Fig. 2. FGFR2 but not FGFR1 partitions into detergent-insoluble lipid rafts from OL and myelin but not from astrocytes.
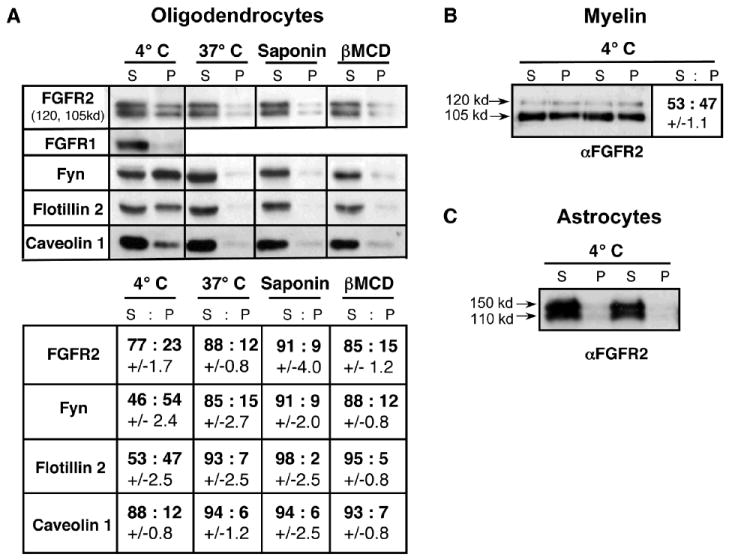
(A) OL were extracted with 1% TX-100 at 4°C, 37°C, or after saponin or βMCD treatment, separated into soluble supernatant (S) and insoluble pellet (P) fractions and analyzed by immunoblotting with anti-FGFR2, anti-FGFR1, and other markers of lipid rafts (Fyn, Flotillin2, Caveolin1). Upon disruption of lipid rafts by various treatments (37°C, saponin, βMCD), a decrease of FGFR2 in the pellet was observed. FGFR1 in OLs did not partition into the insoluble fraction. The relative distribution of FGFR2 and other proteins in detergent-insoluble and -soluble fractions was quantified by densitometric scanning of the protein bands. (B) Purified myelin also was extracted with 1% TX-100 at 4°C and analyzed similarly for the relative distribution of FGFR2 into (S) and (P) fractions. Duplicate samples are shown. Both 120-kd and 105-kd isoforms of FGFR2 partitioned into detergent-insoluble lipid raft fractions, the proportions being ~25% in OLs and ~50% in myelin (A and B). (C) FGFR2 in astrocytes did not partition into the insoluble fraction. Duplicate samples are shown. Standard error, N=3-6.
