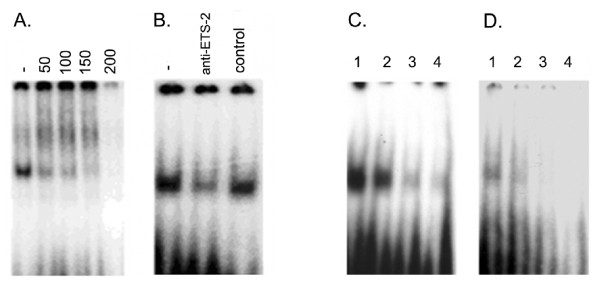
Figure 8.
Electrophoretic mobility shift assays demonstrating that the putative ETS site and the repeated elements in the boPAG-2 promoter are capable of binding proteins in trophoblast nuclear extracts. A. Competition of ETS-2 binding activity (20 μg protein) with cold ETS-2 probe. Nuclear extracts were incubated with 1 μL of 50 pmol probe, in the absence or presence of the indicated molar excess of cold probe (indicated along the top). B. The ETS-2 complex composition was examined by depleting ETS-2 with an antibody specific to ETS-2. Preincubation of the ETS antibody with the nuclear extracts followed by binding reaction with the probe resulted in specific dissociation of the complex. Control: normal rabbit serum. C and D. Competition assays indicating specificity of association of, as yet unknown, TFs capable of binding to the unique bovine tandem repeats, BR1(C) and BR2 (D). Lane 1: labeled probe and nuclear extract; Lane 2: same as lane 1 except for addition of a 50-fold molar excess of unlabeled double-stranded oligonucleotide; Lane 3: 250-fold molar excess of unlabeled probe; Lane 4: 500-fold molar excess.