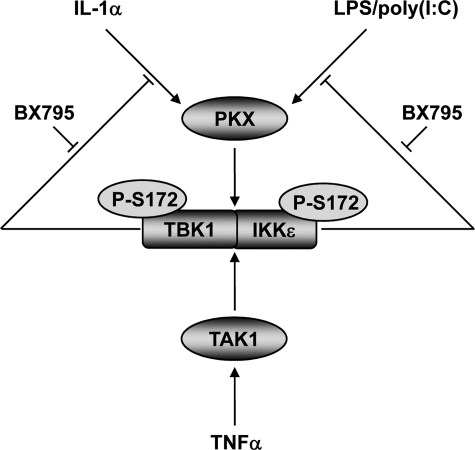
FIGURE 10.
Model for the activation and feedback control of TBK1 and IKKε by proinflammatory stimuli. The binding of IL-1α, LPS, and poly(I:C) to their respective receptors induces the activation of an unidentified protein kinase (PKX). PKX subsequently phosphorylates TBK1 and IKKε at Ser-172, thereby triggering their activation. TBK1 and IKKε can then phosphorylate substrates such as the transcription factor IRF3. As shown in this study, TBK1 and IKKε also exert a negative feedback control on their activation by IL-1α, LPS, and poly(I:C) presumably by phosphorylation and inhibition of an upstream component of the pathway and/or by activating a Ser-172 protein phosphatase (not illustrated). The TNFα-stimulated activation of TBK1 and IKKε appears to require a separate or additional protein kinase that is at least partially dependent on TAK1 activity, and this arm of the pathway is not subject to feedback control.