Abstract
Autotaxin or NPP2 (nucleotide pyrophosphatase/phosphodiesterase 2) is a secreted lysophospholipase-D that promotes metastasis and tumor growth by its ability to generate lysophosphatidic acid. Considerable evidence suggests that inhibitors of NPP2 can be used as a novel therapy for the treatment of cancer. Although most attention is currently directed toward the development of inhibitors of the catalytic site, we have explored whether NPP2 can also be targeted through its non-catalytic nuclease-like domain. We demonstrate here that the catalytic and nuclease-like domains are covalently linked by an essential disulfide bridge between Cys413 and Cys805. Within the nuclease-like domain, residues 829–850 are involved in the secretion of NPP2, and Lys852 is required for the expression of catalytic activity. These data show that the nuclease-like domain is crucial for catalysis by NPP2 and is a possible target to generate inhibitors.
NPP2 3 or autotaxin is a secreted lysophospholipase-D that acts in a paracrine or autocrine manner. The major substrate of NPP2 is lysophosphatidylcholine, which is converted into choline and lysophosphatidic acid. The latter promotes signaling through specific G-protein-coupled receptors that stimulate cell proliferation, differentiation, and motility (1). NPP2 functions in processes as diverse as the homing of lymphocytes, blood vessel formation, and wound healing but also promotes tumorigenesis (2–6). The metastasis-enhancing properties of NPP2 have been attributed to its ability to promote the invasive properties of cancer cells and to stimulate angiogenesis. Importantly, NPP2 is highly expressed by various cancers including breast carcinoma (7), Hodgkin lymphoma (8), and glioblastoma multiforme (9), and this correlates with an increased metastasis and angiogenesis. Therefore, NPP2 is considered to be an attractive target for a novel anticancer therapy, in particular because it acts extracellularly, and interfering drugs thus do not need to be cell-permeable (10).
NPP2 is one of the seven mammalian members of the NPP-type family of ectophosphodiesterases, belonging to the superfamily of phospho-/sulfo-coordinating metalloenzymes (11). These enzymes all have a structurally related catalytic domain and the same catalytic mechanism but show a different substrate specificity. For example, NPP1 only recognizes nucleotide substrates, whereas NPP2 preferentially hydrolyzes lysophospholipids. Interestingly, NPP1–3 share a C-terminal non-catalytic domain, commonly referred to as the nuclease-like domain (NLD) (12). The latter is related to DNA/RNA-nonspecific endonucleases but lacks key residues that are necessary for activity and is therefore believed to have a structural or regulatory role. It is not known whether the NLD acts as a positive or negative regulator of NPP activity, but swapping experiments of the NLD between NPP1 and NPP2 suggested that it harbors isoform-specific determinants for catalysis (13).
To examine the therapeutic potential of NPP2, in particular for the treatment of cancer, a number of small molecule inhibitors have been developed (14, 15). However, these inhibitors are all directed against the catalytic site and may therefore also interfere with other phospho-/sulfo-coordinating metalloenzymes. We report here that the NLD is essential for the expression of catalytic activity and can be used as an alternative target to inhibit NPP2.
MATERIALS AND METHODS
NPP2 Constructs—Wild-type NPP2 (WT NPP2) was obtained by cloning rat NPP2 into the EGFP-N1 vector (Clontech). The EGFP tag at the C terminus was replaced by a c-Myc tag, and a FLAG epitope was introduced after the furin cleavage site to create an N-terminally tagged protein (16). Deletion as well as partial reconstitution of the nuclease-like domain of NPP2 was obtained by PCR with wild-type NPP2 as template and using 3′-GTCGACGAGCAGAAGCTGATCTC-5′ as a sense primer in combination with an antisense primer starting at the residues indicated in the constructs. The last 12 amino acids of NPP2 were replaced by those of NPP1 by a PCR using 3′-ATCTTCAGCCAAGAAGACGTCGACGAGCAGAAGCTGATCTCC-5′ as a sense primer and 3′-TGGCAAATGTGTTTTCAAGGTCAGAATTTCCGAATAGCTACGG-5′ as an antisense primer. The underlined nucleotides correspond to the last 12 amino acids of NPP1. In the sense primer, these are followed by a sequence that anneals to the c-Myc affinity tag. In the antisense primer, the NPP1 nucleotides are preceded by base pairs coding for residues 842–850 of NPP2. These primers enable reading over the last 12 amino acids of NPP2 and directly replacing them for those of NPP1. Site-directed mutagenesis of the LKT triad was performed using the QuikChange™ kit (Stratagene). The same strategy was used for mutation of the Cys residues in the catalytic and the nuclease-like domain as well as introduction of the tobacco etch virus (TEV) cleavage site. All constructs were verified by sequencing. The sequence of all primer sets used is available upon request.
Cell Culture—HEK293T cells were maintained at 37 °C under a humidified atmosphere containing 5% CO2 in Dulbecco's modified Eagle's medium, supplemented with 10% (v/v) heat-inactivated fetal bovine serum, glucose (4.5 g/liter), penicillin (100 units/ml), and streptomycin (100 μg/ml). Cells were transiently transfected at 30–40% confluency using linear polyethyleneimine of 25 kDa (Polysciences, Warrington, PA). The medium was collected 72 h after transfection and cleared by centrifugation at 1000 g for 15 min. The cells were harvested at the same time point, washed once in phosphate-buffered saline (PBS) and lysed in 50 mm Tris/HCl at pH 7.5, 0.5 mm phenylmethylsulfonyl fluoride, 0.5 mm benzamidine, 150 mm NaCl, and 1% (v/v) Triton X-100. The supernatant obtained after ultracentrifugation (45 min at 100,000 g) was used as “cell lysate.” Aliquots of the cell lysates and media were used for immunoblot analysis and the assay of NPP2-associated activities.
Assay of NPP2 Activities—Lysophospholipase-D activities were measured by incubating aliquots of media or cell lysates with the substrate lysophosphatidyl choline (14:0) at 37 °C in a total volume of 40 μl. Subsequently, the released choline was quantified spectrophotometrically at 540 nm after incubation for 5 min with 50 μl each of the peroxidase reagent (50 mm Tris at pH 9.0, 2 mm N-ethyl-N-(2-hydroxy-3-sulfoproryl)-3-methylaniline, 5 units/ml peroxidase, and 0.01% (v/v) of Triton X-100) and of the choline oxidase reagent (50 mm Tris at pH 9.0, 2 mm aminoantipyrine, choline oxidase (5 units/ml), and 0.01% Triton X-100) (17).
Nucleotide phosphodiesterase activities were determined from the release of p-nitrophenolate from p-nitrophenyl thymidine 5′-monophosphate (17). Briefly, the samples were incubated at 37 °C with 5 mm of the substrate, 5 mm CaCl2, 5 mm MgCl2, and 100 mm Tris/HCl at pH 9.0 in a total volume of 35 μl. The reaction was stopped by the addition of 100 μl of 6% (v/v) trichloroacetic acid. Subsequently, the mixture was neutralized with NaOH, and p-nitrophenolate was quantified colorimetrically at 405 nm.
Electrophoresis and Immunoblotting—Proteins were separated by SDS-PAGE using 4–12% Bis-Tris gels (Invitrogen), transferred to polyvinylidene difluoride membranes (GE Healthcare), and subjected to 60 V for 2 h in 192 mm glycine, 25 mm Tris, and 10% methanol (pH 8.6). After blotting, nonspecific binding sites were blocked with 3% bovine serum albumin (Serva) or 5% milk in PBS with 0.1% Tween 20. Subsequently, membranes were incubated with anti-Myc (clone 9E10) monoclonal antibodies or anti-FLAG polyclonal antibodies (Sigma), respectively. After overnight incubation, proteins were visualized using horseradish peroxidase goat anti-mouse or swine anti-rabbit IgG (Dako) and enhanced chemiluminescence (PerkinElmer Life Sciences).
Immunofluorescence—HEK293 cells were seeded in 4-well chambers at a density of 30,000 cells/well. Cells were transfected using the FuGENE™ 6 transfection reagent (Roche Applied Science). After 24 h, the medium was removed, and the cells were washed twice in PBS. Subsequently, the cells were fixed in 2% paraformaldehyde for 10 min and permeabilized with 0.5% Triton X-100 for 10 min. After several rinses in PBS, nonspecific binding was reduced by washing the cells with 3% bovine serum albumin in PBS for 20 min. Following this blocking step, the cells were incubated with anti-Myc (clone 9E10) to visualize NPP2-(1–594) and anti-Spca (secretory pathway calcium ATPase) antibodies (kindly donated by Dr. F. Wuytack, University of Leuven, Belgium) to mark the Golgi apparatus. Expression of the autofluorescent NPP2-EGFP was monitored directly. Cells were washed again and incubated for 60 min with a secondary antibody, i.e. anti-mouse Dylight 549 (Pierce) for NPP2-(1–594) and anti-rabbit Alexa Fluor 488 (Molecular Probes/Invitrogen) to stain the Golgi. After washing in PBS, fluorescence was observed with an LSM 510 Axiovert 100M laser-scanning microscope (Zeiss).
TEV Cleavage of NPP2—In a total volume of 75 μl, the NPP2 TEV mutant was incubated overnight at 30 °C with 1 unit of recombinant TEV protease (Invitrogen) in the presence of 0.1 mm dithiothreitol (DTT), 5 mm citrate, 200 mm NaCl, 10 μm ZnCl2, and 20 mm Tris/HCl at pH 7.4. Cleavage was analyzed by immunoblotting.
Identification of the Disulfide Bridge between Cys413 and Cys805—To study the possible disulfide bridge between Cys413 and Cys805, Cys784 was mutated into Ala in an NPP2 construct with all glycosylation sites mutated, except for Asn524 (17). This Myc-tagged NPP2 mutant was transiently transfected in HEK293T cells that were kept in conditioned medium containing 5% fetal bovine serum. 72 h after transfection, the medium was collected, cleared by centrifugation (15 min at 1000 × g), and incubated overnight with anti-c-Myc covalently bound to CNBr-activated Sepharose. Retained proteins were eluted with 2 m NaCl in 20 mm Na2HPO4 at pH 11.0. The purity of the protein was assessed by SDS-PAGE and Coomassie Blue staining. Next, 10 μg of pure protein was proteolyzed by 1 unit of sequencing-grade trypsin (Roche Applied Science) in 200 mm ammonium bicarbonate, pH 8.5, at 37 °C. Subsequently, the digested protein was subjected to an overnight incubation at 4 °C in the presence or in the absence of 150 mm DTT. The resulting mixture was loaded on a C18 pipette tip (Perfect Pure, Eppendorf) for cleanup. The eluate was evaporated and reconstituted in 1 μl of α-cyano-4-hydroxycinnamic acid matrix solution (3.5 mg/ml α-cyano-4-hydroxycinnamic acid, 50% acetonitrile, 0.1% trifluoroacetic acid). Matrix-assisted laser desorption/ionization-time of flight/time of flight analysis was executed on an ABI 4800 instrument (Applied Biosystems, Foster City, CA), operated in reflectron mode.
RESULTS
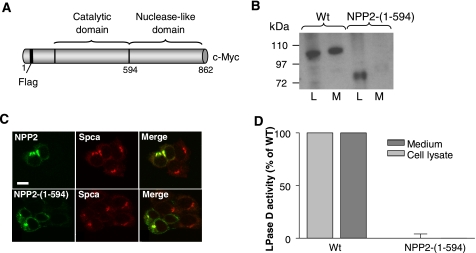
The Nuclease-like Domain of NPP2 Is Required to Generate a Catalytically Active Enzyme—To explore the role of the NLD, we first examined the effect of its deletion on the synthesis, secretion, and catalytic activity of NPP2. When transiently expressed in HEK293T cells, WT NPP2 was present in both the cell lysate and the culture medium, as detected by immunoblotting (Fig. 1B). NPP2-(1–594), lacking the NLD (Fig. 1A), was also readily detected in the cell lysate but was not recovered in the culture medium (Fig. 1B). Because confocal microscopy showed a clear co-localization of both WT NPP2 and NPP2-(1–594) with the calcium ATPase Spca (Fig. 1C), a marker of the Golgi apparatus, these data suggest that the NLD harbors determinants for the transport of NPP2 from the Golgi to the extracellular space. In addition, the NLD appears to be required for the expression of catalytic activity because NPP2-(1–594) in the cell lysates did not exhibit any lysophospholipase-D (Fig. 1D) or nucleotide phosphodiesterase activities (not shown).
FIGURE 1.
The NLD is necessary to generate secreted and active NPP2. A, schematic representation of WT NPP2 showing the catalytic and nuclease-like domains as well as the affinity tags that mark each domain. The FLAG tag will be N-terminal following the processing of NPP2 as a pre-pro-enzyme (16). B, HEK293T cells were transiently transfected with WT NPP2 or NPP2-(1–594). After 72 h, the cell lysate (L) and the medium (M) were analyzed by immunoblotting with anti-Myc antibodies. C, 24 h after transfection, HEK293T cells were fixed and permeabilized. NPP2, NPP2-(1–594), and the Golgi apparatus were visualized by confocal microscopy using anti-Myc antibodies or anti-Spca antibodies, respectively. D, using the activities of mock-transfected cells as blanks, the lysophospholipase-D (LPase D) activity of equal amounts (see A) of WT (medium and cell lysate) and NPP2-(1–594) (Cell lysate) was determined. The bar diagram represents the means of three independent experiments (± S.E.).
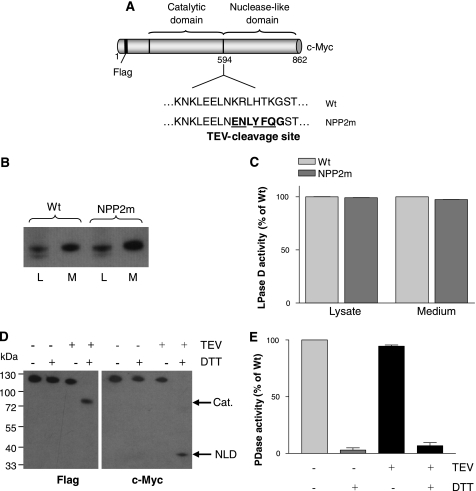
It could be argued that NPP2-(1–594) is catalytically inactive because the NLD is required for a post-translational maturation step of the catalytic domain. Alternatively, the NLD can be an intrinsic part of the catalytic fold. To differentiate between these possibilities, we generated a catalytically active NPP2 with an N-terminal FLAG tag, a C-terminal Myc tag, and a specific cleavage site for the TEV protease (18, 19) introduced in the linker region between the catalytic domain and the NLD (Fig. 2A). This made it possible to examine the effect of the proteolytic removal of the NLD on the catalytic activity. It was verified that the resulting mutant, further referred to as NPP2m, was properly secreted (Fig. 2B) and displayed a normal catalytic activity (Fig. 2C). Incubation with the TEV protease did generate the expected FLAG-tagged N-terminal fragment, comprising the catalytic domain and the Myc-tagged NLD, as detected by immunoblotting (Fig. 2D). However, these fragments only migrated as separate polypeptides during SDS-PAGE when the sample buffer was supplemented with DTT, indicating that the catalytic and nuclease-like domains remained covalently linked by one or more disulfide bridges following TEV proteolysis. Cleavage of NPP2m with the TEV protease did not affect its nucleotide phosphodiesterase (Fig. 2E) or lysophospholipase-D activities (not shown). However, the catalytic activity was completely lost in the presence of DTT, both before and after incubation with the TEV protease, suggesting that the activity depends on one or several disulfide bridges. Our finding that the catalytic and nuclease-like domains remained linked by (a) disulfide bond(s) following TEV cleavage precluded a clear interpretation on the role of the NLD for catalysis at this stage.
FIGURE 2.
Introduction of a TEV cleavage site between the catalytic and the nuclease-like domain of NPP2. A, schematic representation showing the introduction of the TEV cleavage site between the catalytic and the nuclease-like domain, creating NPP2m. B, equal loading of WT NPP2 and NPP2m in the cell lysate (L) and medium (M) was verified using c-Myc antibodies. C, graph showing the corresponding lysophospholipase-D (LPase D) activities (n = 3, means ± S.E.). D, cleavage of NPP2m by the TEV protease was monitored by immunoblotting in the presence or absence of 10 mm DTT. Anti-FLAG and anti-Myc antibodies were used to visualize the catalytic (Cat.) and nuclease-like (NLD) domains, respectively. E, the phosphodiesterase (PDase) activity of NPP2m in the conditions shown in D was analyzed in triplicate. The results are represented in the bar diagram (means ± S.E.).
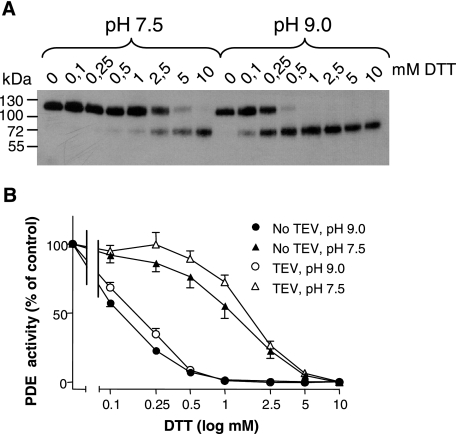
The essential disulfide bridge(s) in NPP2 could be intradomain and/or interdomain bond(s). Initial evidence that they involved bonds between the catalytic and nuclease-like domains came from observations that the concentration of DTT needed to dissociate these domains after TEV-mediated proteolysis (Fig. 3A) corresponded well to the concentration that caused an inactivation of NPP2m (Fig. 3B). The same correspondence was seen at a more alkaline pH (9 versus 7.5), which increased the sensitivity to DTT about 10-fold, consistent with the higher prevalence of the reactive thiolate form of DTT at alkaline pH.
FIGURE 3.
Effect of DTT on the activity and TEV-mediated cleavage of NPP2m. A, after a 30-min preincubation with increasing concentrations of DTT at pH 7.5 or pH 9.0, the TEV cleavage of NPP2 was analyzed by immunoblotting with anti-FLAG antibodies, visualizing the catalytic domain. B, the graph shows the phosphodiesterase activity corresponding to the signals detected in A. As a control, the same experiment was performed with NPP2m that was not treated with the TEV protease. The results are expressed as a percentage of the activity in the absence of DTT (means ± S.E., n = 3).
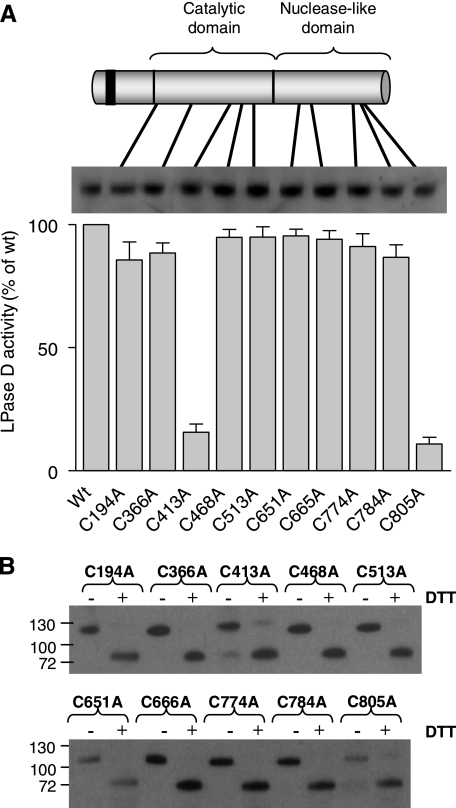
Mapping of an Essential Interdomain Disulfide Bond—To identify the cysteines that are essential for the expression of catalytic activity, all 11 Cys residues in the catalytic and nuclease-like domains of NPP2m were mutated individually to Ala (Fig. 4A). Except for NPP2-C350A (not illustrated), all mutants were expressed well and could be detected in the culture medium. Analysis of the lysophospholipase-D activity of equal amounts of these Cys to Ala mutants showed that they all displayed a similar specific activity, except for C413A and C805A, which were only weakly active. Because Cys413 and Cys805 are located in the catalytic and nuclease-like domains, respectively, we hypothesized that they form an essential disulfide bridge between both domains. However, these data do not rule out the existence of additional intradomain or interdomain disulfide bridges that are, however, not essential for the expression of catalytic activity. Actually, following TEV proteolysis of the Cys to Ala mutants, including C413A and C805A, they only migrated as cleaved products in the presence of DTT, confirming the presence of at least one additional interdomain S–S bond (Fig. 4B).
FIGURE 4.
Cys413 and Cys805 are essential for the catalytic activity of NPP2. A, the Cys residues in the catalytic and the nuclease-like domains were individually mutated into an Ala and transiently transfected in HEK293T cells. After 72 h, the lysophospholipase-D (LPase D) activity of equal amounts of these mutants was determined (means ± S.E., n = 3). B, after treatment with the TEV protease, the media containing the Cys to Ala mutants were analyzed in the presence or absence of 10 mm DTT by immunoblotting with anti-FLAG antibodies, visualizing the catalytic domain.
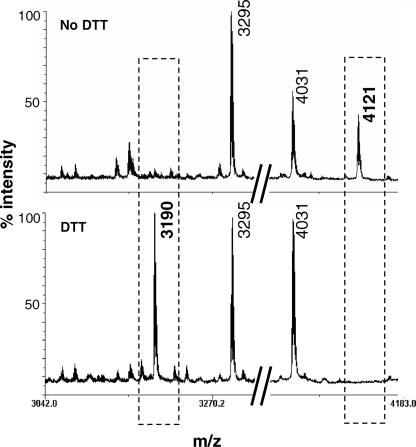
To obtain more direct evidence for a disulfide bond between Cys413 and Cys805, we used mass spectrometry. Initially, this analysis was hampered by the fact that Cys413 was recovered in a heavily glycosylated tryptic fragment. In addition, the tryptic fragment containing Cys805 also harbored Cys784, possibly involved in another disulfide linkage. Taken together, the hall-mark fragment for evaluation of the disulfide linkage between Cys413 and Cys805 displayed a molecular weight not amenable to reflectron MALDI-TOF/TOF. To circumvent these difficulties, we used an active NPP2 variant with mutated glycosylation sites (Asn to Ala), except for Asn524, whose glycosylation is essential to adopt an active conformation (17). In this glycosylation mutant, Cys784 was also converted into an Ala. Following purification and trypsinolysis under non-reducing conditions, the mass spectrum of this NPP2 variant displayed a peak of m/z 4121, corresponding to the mass of the disulfide-linked peptides (406TIIAALTCK414)-(784ADGPLSVSSFILPHRPDNDESCASSEDESK814) (Fig. 5, upper panel, right box). The identity of this species was confirmed by MS/MS (not shown), producing clear b12, b17, and b19 fragments of 784ADGPLSVSSFILPHRPDNDESCASSEDESK814. The m/z 4121 peak completely disappeared after a treatment with DTT (Fig. 5, lower panel, right box). In addition, a peak of m/z 3190, absent in the non-reduced sample and corresponding to the molecular weight of 784ADGPLSVSSFILPHRPDNDESCASSEDESK814, was detected (Fig. 5, left box). The identity of this species was confirmed by MS/MS of the m/z 3190 peak, producing b12, b17, and b19 fragments and an additional y28 fragment (not shown). The peptide harboring Cys413 was not recovered under reducing conditions, probably due to the low content of ionizable amino acids in this fragment. Collectively, these data identified a disulfide bridge between Cys413 and Cys805 that covalently anchors the catalytic and nuclease-like domains of NPP2 and that is essential for the expression of catalytic activity.
FIGURE 5.
Mapping of a disulfide bridge between Cys413 and Cys805. A mutant NPP2 construct lacking all glycosylation sites, except for Asn524 and with Cys784 mutated into Ala, was purified from the conditioned medium of HEK293T cells. After trypsinization, peptides were analyzed by matrix-assisted laser desorption/ionization-time of flight/time of flight analysis. The upper panel shows the spectrum obtained in the absence of DTT. The lower panel is obtained after reduction with 150 mm DTT. The peaks marked by the left or right box correspond to the peptide containing Cys805 and the disulfide bonded peptides, respectively. The molecular weight (m/z) of each peak is indicated.
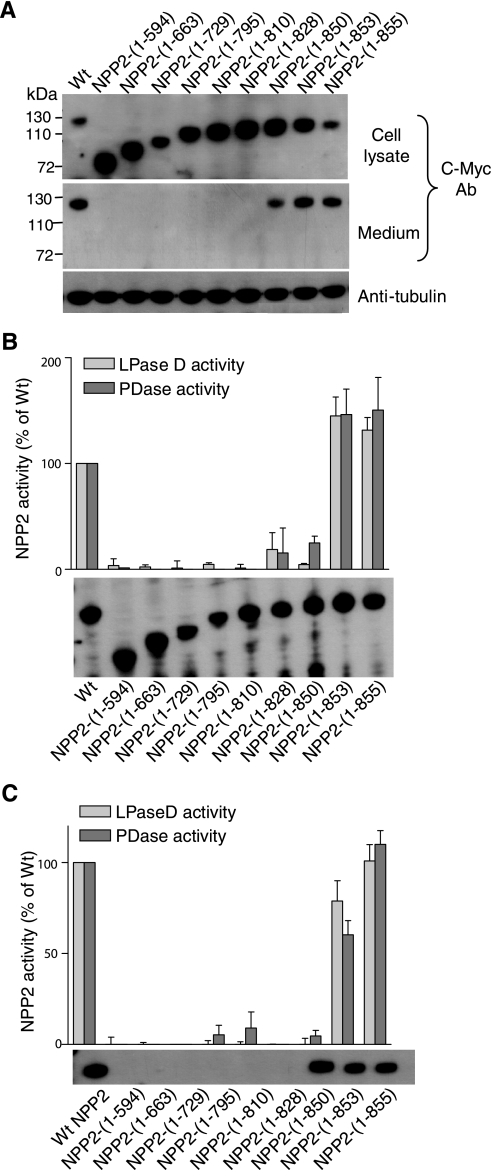
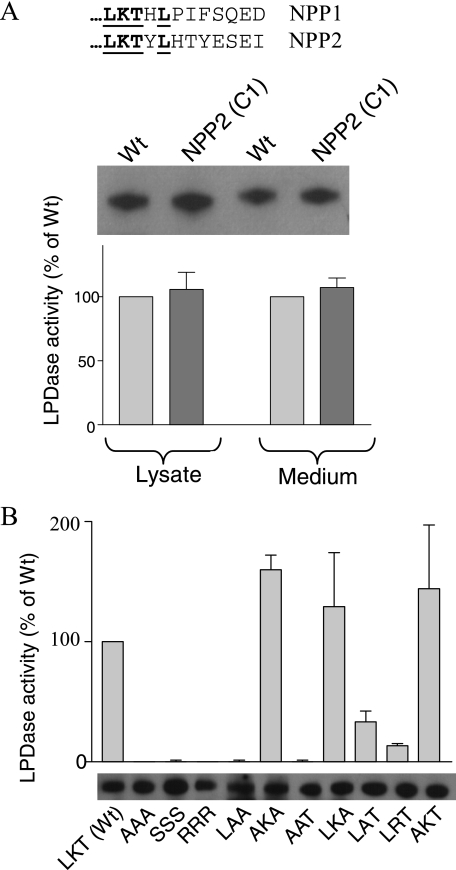
Identification of an Essential Lysine in the C Terminus of the NLD—As an independent and alternative strategy to study the contribution of the NLD to the secretion and activity of NPP2, we used a stepwise rescue approach starting from the inactive, non-secreted NPP2-(1–594). C-terminal extensions of up to 139 residues, as in NPP2-(1–828), did not yield secreted proteins (Fig. 6A). This was unexpected because NPP2-(1–828) contains all the residues that are glycosylated (Asn524) or form the essential disulfide bond (Cys413 and Cys805). Because NPP2-(1–850) was recovered in the culture medium (Fig. 6A), these data therefore suggested that residues 829–850 are essential for the secretion of NPP2. Intriguingly, NPP2-(1–850) was only marginally active, both in the cell lysate (Fig. 6B) and in the medium (Fig. 6C), suggesting that the C-terminal 12 residues of NPP2, although not needed for secretion, are essential to obtain a fully active enzyme. Additional C-terminal extensions, as in NPP2-(1–853) and NPP2-(1–855), yielded fully active enzymes, hinting at an essential role for residues 851–853, comprising the triad Leu-Lys-Thr (LKT). Because the C termini of NPP1 and NPP2 are very different, except for this LKT triad (Fig. 7A), we also generated NPP2 with the C-terminal 12 residues of NPP1. This chimeric construct was secreted and fully active, providing additional evidence for an essential role of the LKT triad for catalysis. Finally, we explored the effects of mutagenesis of these three residues, either individually or combined, on the catalytic activity (Fig. 7B). The mutation of the LKT triad into AAA, SSS, or RRR yielded completely inactive NPP2 variants. Also, mutation of Lys852 into an Ala or Arg strongly reduced the catalytic activity, but the replacement of Leu851 or Thr853 by an Ala did not affect the catalytic activity. Collectively, these studies identified Lys852 as a residue that is essential for the expression of catalytic activity.
FIGURE 6.
Mapping of the NLD fragments that are needed for secretion and catalytic activity. A, using specific primer sets, a series of NPP2 proteins with varying NLD fragments was obtained. Expression and secretion of these constructs was monitored by immunoblotting with c-Myc antibodies (Ab). Tubulin was used as an internal control for equal loading. Next, immunoblotting was repeated to determine equal amounts of these proteins in the cell lysate (B) and the medium (C), and these volumes were used to measure the corresponding lysophospholipase-D (LPase D) activities. The bar diagrams show the means of three independent experiments (±S.E.). PDase, phosphodiesterase.
FIGURE 7.
Identification of Lys852 as an essential residue for the expression of activity. A, alignment of the last 12 amino acids of NPP1 and NPP2. The bold underlined sequence indicates the amino acids that are conserved between both proteins. Using a deletion-insertion strategy, the last 12 amino acids of NPP2 were exchanged for those of NPP1, yielding NPP2-(C1). Equal amounts of WT NPP2 and NPP2-(C1) were estimated by immunoblotting with c-Myc antibodies. The same volumes were used to study the lysophospholipase-D activity (n = 3, mean ± S.E.). B, the residues of the LKT-triad were mutated as indicated, and the corresponding mutants in the medium (subset) were assayed for their lysophospholipase-D (LPase D) activities. The results are represented as means (n = 3) ± S.E.
DISCUSSION
The introduction of a TEV cleavage site between the catalytic and nuclease-like domains of NPP2 has enabled us to show that these domains are covalently linked by at least two disulfide bonds (Figs. 2 and 4). Only one of these bridges turned out to be indispensable for the expression of catalytically active NPP2 and was unequivocally mapped by site-directed mutagenesis (Fig. 4) and mass spectrometry (Fig. 5) to Cys413 and Cys805. An essential covalent association between these domains was unexpected and suggests that the NLD somehow actively contributes to catalysis. Consistent with this notion, we could identify by mutagenesis studies Lys852 as a residue that is needed for enzymatic activity (Figs. 6 and 7). Remarkably, even the conservative replacement K852R resulted in a largely inactive enzyme (Fig. 7B). The exact role of the NLD in catalysis is not understood, but we speculate that this non-catalytic domain could be involved in substrate binding and/or in product release. Consistent with a role in substrate binding, Parrill et al. (20) failed to dock lysophosphatidylcholine species onto their model of the catalytic core of NPP2, suggesting that substrate-binding residues lie outside the catalytic domain. This notion is also supported by the observation that NPP2 with the NLD of NPP1 is catalytically inactive (13), suggesting that the NLDs harbor isoform-specific substrate-specifying determinants. As for a possible role of the NLD in product release, it is worthy of note that this represents the rate-limiting step of catalysis by NPPs (21). Interestingly, the nuclease-like domain of the DNA repair protein RecC forms an aperture through which one strand of the newly split DNA is guided (22). We speculate that a similar fold could guide product release in NPP2.
We have also found that residues 829–850 of the NLD are involved in the secretion of NPP2 (Fig. 6). Because NPP2 without the NLD accumulates in the Golgi (Fig. 1), this suggests that the NLD harbors determinants for its secretion. In this respect, it cannot be excluded that the NLD has an indirect role and is required for a post-translational maturation step, such as glycosylation or the rearrangement of S–S bonds, that needs to be completed before NPP2 can be secreted.
In conclusion, we have shown here that 1) the catalytic and nuclease-like domains of NPP2 are linked by an essential disulfide bond, 2) residues 829–850 of the NLD are necessary for the secretion of NPP2, and 3) the C-terminal Lys852 is essential for catalysis. Our study shows that the NLD domain can be targeted to generate novel inhibitors of the lysophospholipase-D activity of NPP2.
This work was supported by grants from the Fund for Scientific Research–Flanders (Grant G.0524.06), a Flemish Concerted Research Action, and the Prime Minister's office (Grant IAP/V-05).
Footnotes
The abbreviations used are: NPP, nucleotide pyrophosphatase/phosphodiesterase; NLD, nuclease-like domain; WT, wild type; TEV, tobacco etch virus; PBS, phosphate-buffered saline; DTT, dithiothreitol; EGFP, enhanced green fluorescent protein; Bis-Tris, 2-(bis(2-hydroxyethyl)amino)-2-(hydroxymethyl)propane-1,3-diol.
References
- 1.Choi, J. W., Lee, C. W., and Chun, J. (2008) Biochim. Biophys. Acta 1781 531-539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mazereeuw-Hautier, J., Gres, S., Fanguin, M., Cariven, C., Fauvel, J., Perret, B., Chap, H., Salles, J. P., and Saulnier-Blache, J. S. (2005) J. Investig. Dermatol. 125 421-427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kanda, H., Newton, R., Klein, R., Morita, Y., Gunn, M. D., and Rosen, S. D. (2008) Nat. Immunol. 9 415-423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nakasaki, T., Tanaka, T., Okudaira, S., Hirosawa, M., Umemoto, E., Otani, K., Jin, S., Bai, Z., Hayasaka, H., Fukui, Y., Aozasa, K., Fujita, N., Tsuruo, T., Ozono, K., Aoki, J., and Miyasaka, M. (2008) Am. J. Pathol. 173 1566-1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tanaka, M., Okudaira, S., Kishi, Y., Ohkawa, R., Iseki, S., Ota, M., Noji, S., Yatomi, Y., Aoki, J., and Arai, H. (2006) J. Biol. Chem. 281 25822-25830 [DOI] [PubMed] [Google Scholar]
- 6.van Meeteren, L. A., Ruurs, P., Stortelers, C., Bouwman, P., van Rooijen, M. A., Pradere, J. P., Pettit, T. R., Wakelam, M. J., Saulnier-Blache, J. S., Mummery, C. L., Moolenaar, W. H., and Jonkers, J. (2006) Mol. Cell Biol. 26 5015-5022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang, S. Y., Lee, J., Park, C. G., Kim, S., Hong, S., Chung, H. C., Min, S. K., Han, J. W., Lee, H. W., and Lee, H. Y. (2002) Clin. Exp. Metastasis 19 603-608 [DOI] [PubMed] [Google Scholar]
- 8.Baumforth, K. R., Flavell, J. R., Reynolds, G. M., Davies, G., Pettit, T. R., Wei, W., Morgan, S., Stankovic, T., Kishi, Y., Arai, H., Nowakova, M., Pratt, G., Aoki, J., Wakelam, M. J., Young, L. S., and Murray, P. G. (2005) Blood 106 2138-2214 [DOI] [PubMed] [Google Scholar]
- 9.Hoelzinger, D. B., Nakada, M., Demuth, T., Rosensteel, T., Reavie, L. B., and Berens, M. E. (2008) J. Neurooncol. 86 297-309 [DOI] [PubMed] [Google Scholar]
- 10.Federico, L., Pamuklar, Z., Smyth, S., and Morris, A. (2008) Curr. Drug Targets 8 698-708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stefan, C., Jansen, S., and Bollen, M. (2005) Trends Biochem. Sci. 30 542-550 [DOI] [PubMed] [Google Scholar]
- 12.Gijsbers, R., Ceulemans, H., and Bollen, M. (2003) Biochem. J. 371 321-330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cimpean, A., Stefan, C., Gijsbers, R., Stalmans, W., and Bollen, M. (2004) Biochem. J. 381 71-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saunders, L. P., Ouellette, A., Bandle, R., Chang, W. C., Zhou, H., Misra, R. N., De La Cruz, E. M., and Braddock, D. T. (2008) Mol. Cancer Ther. 7 3352-3362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Meeteren, L. A., Brinkmann, V., Saulnier-Blache, J. S., Lynch, K. R., and Moolenaar, W. H. (2008) Cancer Lett. 266 203-208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jansen, S., Stefan, C., Creemers, J. W., Waelkens, E., Van, E. A., Stalmans, W., and Bollen, M. (2005) J. Cell Sci. 118 3081-3089 [DOI] [PubMed] [Google Scholar]
- 17.Jansen, S., Callewaert, N., Dewerte, I., Andries, M., Ceulemans, H., and Bollen, M. (2007) J. Biol. Chem. 282 11084-11091 [DOI] [PubMed] [Google Scholar]
- 18.Carrington, J. C., and Dougherty, W. G. (1988) Proc. Natl. Acad. Sci. U. S. A. 85 3391-3395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kapust, R. B., Tozser, J., Fox, J. D., Anderson, D. E., Cherry, S., Copeland, T. D., and Waugh, D. S. (2001) Protein Eng. 14 993-1000 [DOI] [PubMed] [Google Scholar]
- 20.Parrill, A. L., Echols, U., Nguyen, T., Pham, T. C., Hoeglund, A., and Baker, D. L. (2008) Bioorg. Med. Chem. 16 1784-1795 [DOI] [PubMed] [Google Scholar]
- 21.Gijsbers, R., Ceulemans, H., Stalmans, W., and Bollen, M. (2001) J. Biol. Chem. 276 1361-1368 [DOI] [PubMed] [Google Scholar]
- 22.Rigden, D. J. (2005) BMC. Struct. Biol. 5 9. [DOI] [PMC free article] [PubMed] [Google Scholar]