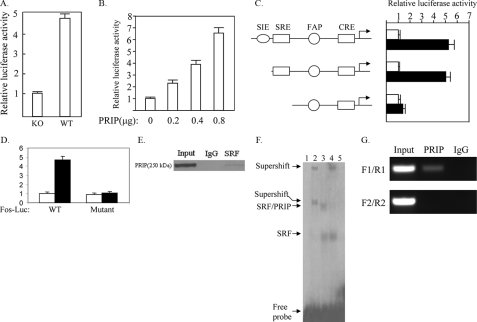
FIGURE 5.
PRIP regulates the FOS promoter activity through SRF. A, decreased activity of the FOS promoter in PRIP-/- tumor cells. PRIP-/- tumor cells (KO) or wild type tumor cells (WT) were transfected with the FOS-Luc reporter and the β-galactosidase expression vector. Luciferase activity was normalized to β-galactosidase activity. B, expression of PRIP increased the activity of the FOS promoter. PRIP-/- cells were transfected with the FOS-Luc reporter, different amounts of PRIP expression vector as indicated, and the β-galactosidase expression vector as the control. C, PRIP activated the FOS promoter through SRE. PRIP-/- cells were transfected with different FOS-Luc reporters, as indicated, control empty vector (white bars), or PRIP expression vector (black bars) and β-galactosidase expression vector. D, confirmation that SRE is required for activation of the FOS promoter by PRIP. Wild type FOS-Luc or FOS-Luc with a mutation in its SRE (Mutant) were transfected into PRIP-/- cells with control empty vector (white bars) or PRIP expression vector (black bars). E, the direct interaction between PRIP and SRF in vivo. The nuclear extract from wild type tumor was prepared and precipitated with anti-SRF or control IgG. The precipitate was subject to Western blot analysis using anti-PRIP. F, PRIP and SRF form a complex on SRE. Gel shift was performed with 32P-labeled SRE, nuclear extracts from Ras-induced tumor cells, and antibodies against SRF or PRIP. Lane 1, no nuclear extract; lane 2, nuclear extract plus anti-SRF; lane 3, nuclear extract; lane 4, nuclear extract plus anti-PRIP; lane 5, nuclear extract plus a 50-fold molar excess of unlabeled competitor SRE oligonucleotide. G, sequential ChIP assay demonstrating PRIP and SRF protein complexes over SRE in the FOS promoter. The first-step ChIP was performed with Ras-induced tumor cells and anti-SRF. The second step ChIP was carried out with the eluates of the initial ChIP, anti-PRIP, and IgG. PCR was performed using primer set F1/R1 and F2/R2, as described above.