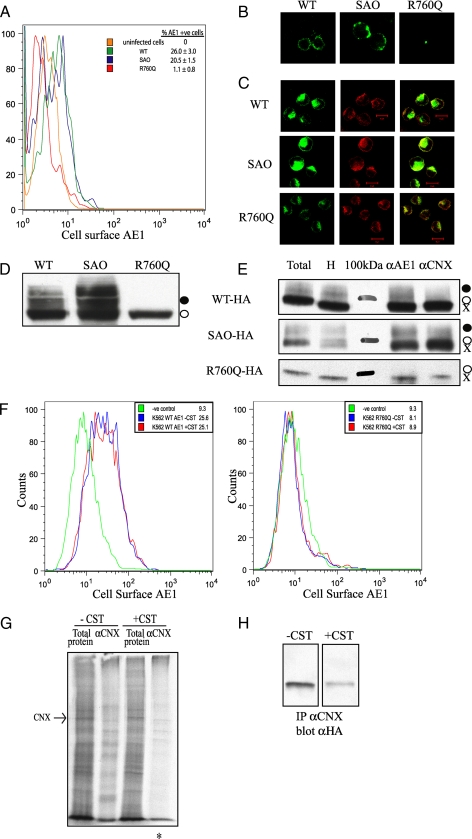
FIGURE 5.
Calnexin is not required for cell-surface expression of membrane glycoproteins in K562 cells. A, flow cytometry shows the cell-surface expression of AE1 and SAO but not R760Q in K562 cells. B, shown is the immunofluorescence of non-permeabilized K562 cells demonstrating cell-surface expression of the wild type (WT) and SAO AE1 and not R760Q. C, shown is the immunofluorescence of permeabilized K562 cells demonstrating intracellular co-localization with calnexin. D, shown is an immunoblot of total protein lysate from K562 cells expressing AE1 constructs containing an HA tag in the third extracellular loop. Expressed AE1 contains primarily the high mannose oligosaccharide (○), with wild type and SAO but not R760Q possessing complex oligosaccharide (•). E, K562 cells expressing AE1 were lysed in 1% digitonin for co-immunoprecipitation with antibodies against AE1 (αAE1) and calnexin (αCNX). Total, total cell extract; H, endoglycosidase H-treated cell extract; 100kDa, 100-kDa protein molecular masss marker. Immunoprecipitated (IP) proteins were run on 8% SDS-polyacrylamide gels and immunoblotted using an anti-HA antibody to detect AE1. F, the inhibition of calnexin binding does not promote cell-surface expression of membrane glycoproteins. Flow cytometry was used to monitor the cell-surface expression of HA-tagged AE1 (left panel, wild type; right panel, HS R760Q), in the presence and absence of 1 mm castanospermine. Geometric mean fluorescence intensities are shown. G, shown is the co-immunoprecipitation of radiolabeled proteins in K562 cells with calnexin in the presence and absence of 1 mm castanospermine. An asterisk indicates less radiolabeled proteins can be associated with calnexin in the presence of castanospermine. H, shown is a co-immunoprecipitation (IP) of HS R760Q AE1 with calnexin in the absence and presence of 1 mm castanospermine.