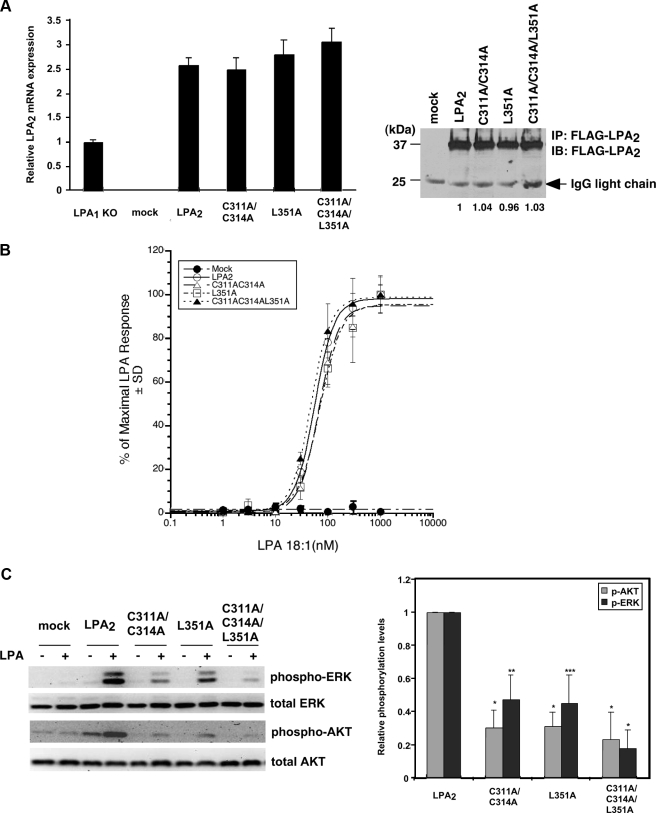
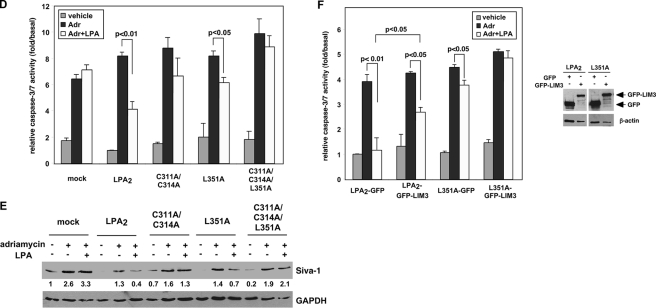
FIGURE 5.
Protein-protein interactions via the CXXC- and/or PDZ-binding motifs do not affect LPA2-mediated Ca2+ response but regulate its chemoprotective effect. A, stable expression of LPA2 or one of the point mutants deficient in binding to the zinc finger proteins and/or PDZ proteins in LPA½ DKO MEFs. Total mRNAs were isolated from LPA1 KO MEFs or one of the LPA½ DKO MEF cell lines (mock, WT LPA2, C311A/C314A, L351A, or C3131A/C314A/L351A). Quantitative real-time reverse transcriptase-PCR was performed to determine the relative expression levels of LPA2 mRNA compared with that expressed in LPA1 KO MEFs and was normalized by the expression of β-actin mRNA. Results show the mean ± S.E. done in triplicates and are representative of two separate experiments. To determine total protein levels of each receptor, FLAG-LPA2 in the whole cell lysates was immunoprecipitated using anti-FLAG M2 monoclonal antibody-conjugated agarose beads and detected with an anti-FLAG rabbit polyclonal antibody. The result shown is representative of five independent experiments. B, mutation of the CXXC motif and/or PDZ-binding motif does not affect LPA2-mediated Ca2+ response. Stable LPA½ DKO MEFs were stimulated with different concentrations of LPA as indicated for 5 min. LPA-induced calcium response was determined as described previously (7). The curves for WT LPA2 and mutants were generated by normalizing the Ca2+ peak at various dilutions to the response elicited by the highest concentration of LPA (1 μm) applied. The values of the mock-transduced MEFs were normalized to that of WT LPA2-transduced MEFs. Data shown are the mean ± S.D. done in triplicates and are representative of two separate experiments. C, mutation of the CXXC motif and/or PDZ-binding motif attenuates LPA2-mediated activation of ERK and AKT. Stable LPA½ DKO MEFs were stimulated with LPA for 10 min. Immunoblotting was performed to detect the levels of phosphorylated and total ERK and AKT (left panel). The intensity of each protein was quantified to determine the relative activation fold of phospho-ERK and AKT by LPA stimulation (right panel). Data shown are the mean ± S.E. of five independent experiments. *, p < 0.001; **, p < 0.01; ***, p < 0.05 versus LPA-stimulated DKO-LPA2 MEFs (Student's t test). D, LPA-mediated protection from adriamycin-induced caspase-3/7 activation is inhibited by mutation of both CXXC- and PDZ-binding motifs. Different LPA½ DKO MEFs transfectants were pretreated with 10 μm LPA in 0.1% fatty acid-free BSA-containing medium for 1 h, followed by addition of 1.7 μm adriamycin for 8 h. Apoptosis was determined by caspase-3/7 activity assay. Data show the mean ± S.E. of four independent experiments. E, mutation of the CXXC motif abrogates LPA2-mediated protection from adriamycin-induced Siva-1 induction. Different LPA½ DKO MEFs were pretreated with 10 μm LPA for 2 h, followed by the addition of 1.5 μm adriamycin for 14 h. Immunoblotting (IB) was performed to detect the expression of Siva-1 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in the whole cell lysates. The relative expression of Siva-1 was compared with that expressed in the mock cells without any treatment and was normalized by the levels of GAPDH. The result shown is representative of three independent experiments. F, inhibition of the CXXC motif-mediated interaction with TRIP6-LIM3 attenuates LPA-mediated protection from adriamycin-induced apoptosis. pEGFP or pEGFP-TRIP6-LIM3 was transiently transfected by electroporation into LPA½ DKO MEFs that expressed WT LPA2 or L351A. Cells were treated with 10 μm LPA for 1 h followed by addition of 1.5 μm adriamycin for 9 h. Caspase-3/7 activity was determined and normalized by protein concentrations in each sample. Data show the mean ± S.E. of three independent experiments. The immunoblot shows the expression of GFP-TRIP6-LIM3, GFP, and β-actin in the whole cell lysates.