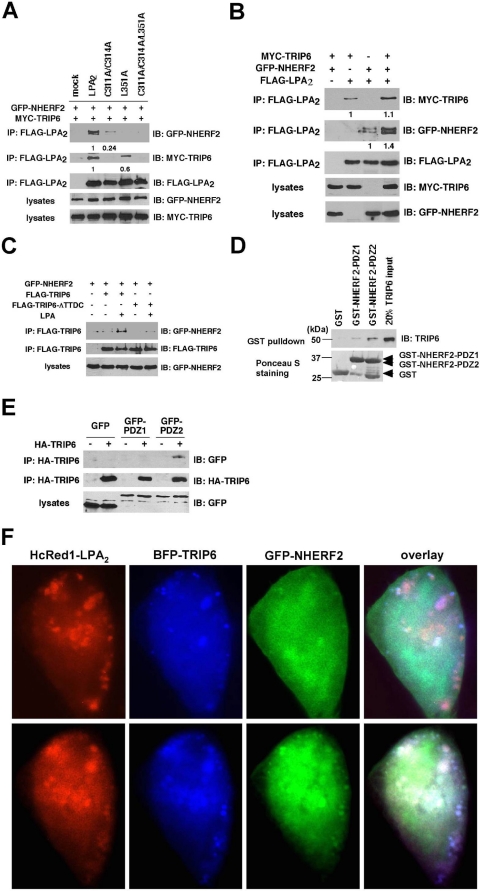
FIGURE 6.
A PDZ-mediated association of TRIP6 and NHERF2 facilitates their interactions with LPA2. A, mutation of the CXXC (PDZ-binding) motif of LPA2 abolishes the binding to TRIP6 (NHERF2) and also reduces the association with NHERF2 (TRIP6). GFP-NHERF2 and MYC-TRIP6 were coexpressed with WT or one of the mutants of FLAG-LPA2 in HEK 293T cells. Co-immunoprecipitation (IP) was performed as described above. The relative levels of co-immunoprecipitated TRIP6 or NHERF2 were quantified and normalized to the immunoprecipitated WT or mutant of LPA2. B, overexpression of TRIP6 enhances association of NHERF2 with LPA2, and vice versa. FLAG-LPA2 was co-expressed with MYC-TRIP6 and/or GFP-NHERF2 in HEK 293T cells as indicated. After stimulation of the cells with 2 μm LPA for 10 min, co-immunoprecipitation was performed. GFP-NHERF2 and MYC-TRIP6 co-immunoprecipitated with FLAG-LPA2 were detected with an anti-GFP antibody and an anti-MYC antibody, respectively. The blot was reprobed with an anti-FLAG antibody to detect the immunoprecipitated LPA2. The relative levels of co-immunoprecipitated TRIP6 or NHERF2 were quantified and normalized to the immunoprecipitated FLAG-LPA2. C, C-terminal TTDC sequences of TRIP6 mediate the binding to NEHRF2. FLAG-TRIP6 or FLAG-TRIP6-ΔTTDC mutant lacking the C-terminal PDZ-binding motif was co-expressed with GFP-NHERF2 in HEK 293T cells. After stimulation of the cells with LPA for 10 min, WT or the ΔTTDC mutant of TRIP6 was immunoprecipitated with anti-FLAG M2 mouse monoclonal antibody-conjugated agarose beads and resolved by SDS-PAGE. Immunoblotting (IB) was performed using the antibodies specific to GFP and the FLAG epitope to detect GFP-NHERF2 and FLAG-TRIP6, respectively. D, TRIP6 binds to the PDZ domain of NHERF2 directly in vitro. Purified recombinant TRIP6 was incubated with GST, GST-NEHRF2-PDZ1, or GST-NHERF2-PDZ2 at 4 °C for 3 h. TRIP6 pulled down by glutathione S-transferase (GST) fusion proteins was detected by immunoblotting using an anti-TRIP6 antibody. The bottom panel shows expression of GST fusion proteins by Ponceau S staining. E, TRIP6 interacts with the PDZ2 but not PDZ1 domain of NHERF2 in cells. HA-TRIP6 was co-expressed with GFP, GFP-NHERF2-PDZ1, or GFP-NHERF2-PDZ2 in HEK 293T cells. TRIP6 in the whole cell lysates was immunoprecipitated with anti-HA mouse monoclonal antibody-conjugated agarose beads and resolved by SDS-PAGE. The immunoblot was probed with an anti-GFP antibody to detect GFP-NHERF2-PDZ2. The blot was reprobed with an anti-HA rabbit antibody to detect the immunoprecipitated HA-TRIP6. Data shown in each figure are representative of two to four independent experiments. HA, hemagglutinin. F, LPA2, TRIP6, and NHERF2 colocalize in cells. Hc-Red1-LPA2 was transiently co-expressed with BFP-TRIP6 and GFP-NHERF2 in LPA½ DKO MEFs. Cells were starved for 1 h, followed by addition of 2 μm LPA for 10 min. Subcellular distribution of these molecules was visualized by fluorescence microscopy.