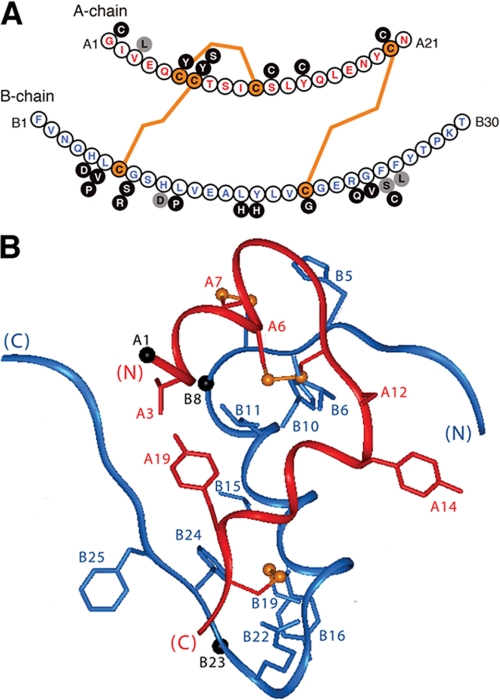
FIGURE 9.
Sites of diabetes-associated mutations in human insulin. A, sequence of human insulin with the A-chain (upper) in red and the B-chain (lower) in blue. Disulfide bridges are shown in orange. Black circles represent mutations associated with neonatal DM due to presumed folding defects in proinsulin (78-81); gray circles represent mutations that permit disulfide pairing in the ER with subsequent circulation of mutant proteins. Whereas substitutions at positions A3, B24, and B25 lead to variant hyperinsulinemias, the B10 substitution interferes with protein trafficking, leading to mutant hyperproinsulinemia (77). B, positions of mutation sites in the T-state of insulin (crystallographic protomer 1 of 2Zn insulin; Protein Data Bank code 4INS) (1). Black spheres indicate Cα atoms of Gly at A1, B8, and B23.