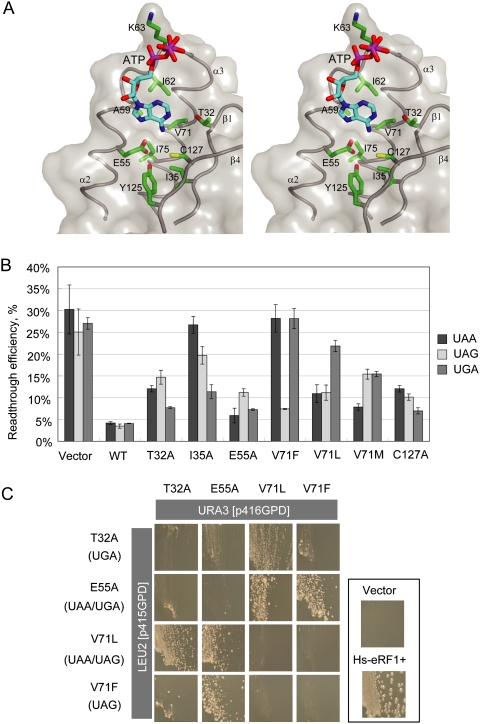
Figure 6.
The binding of ATP to a putative decoding site on domain N of eRF1. (A) Stereo view of the binding pocket. Domain N of eRF1 is shown in transparent gray surface. Residues involved in this binding pocket are labeled and drawn in the green stick model. ATP molecule is shown in the cyan stick model. (B) In vivo data bar graph for selected mutations. (C) Dual-eRF1 complementation assay: Human eRF1 mutant genes that showed prominent single or double stop codon specificities (indicated in parentheses on the left; i.e., loss of omnipotency) were cloned into yeast expression vectors with different selection markers (p416GPD for URA3, p415GPD for LEU2). Tet-off eRF1 strains transformed with dual combinations of vectors were streaked on the marker selective plates supplemented with 10 μg/mL Doxycyclin, a tetracycline derivative, and growth was monitored for 5 d.