Abstract
Background: The accuracy of dietary energy assessment tools is critical to understanding the role of diet in the increasing rate of obesity.
Objectives: The purposes of our study in overweight adolescent boys and girls were 1) to assess the energy reporting bias of diet records against the referent of total energy expenditure (TEE) and 2) to compare the methods of determining energy needs by using measured metabolizable energy intake (MEI) and TEE.
Design: Twenty girls [12–15 y, body mass index (in kg/m2) = 33.0 ± 5] and 14 boys (12–14 y, body mass index = 27.4 ± 4) participated in two 3-wk metabolic balance studies. TEE was measured by using doubly labeled water (TEEDLW), and MEI was measured by bomb calorimetry of composite daily diet, urine, and fecal collections. Food records were collected before each study.
Results: Food records underreported TEEDLW by 35 ± 20%. Underreporting of energy intake was correlated with all macronutrient intake concentrations (g or kcal) (P < 0.0001). A multiple regression model showed that 86.4% of the variance in underreporting error was explained by dietary fat (g), BMI, and sex. The intrasubject CV was 3.9% for TEEDLW and 9.9% for MEI. MEI for weight stability (MEIwtstb) averaged 99 ± 11% of TEE.
Conclusions: The increased underreporting of dietary intake with increasing body weight in teens may explain in part previous reports noting that there has been an increased incidence of obesity, although energy intakes have not appeared to increase. MEIwtstb and TEEDLW gave similar estimates of energy needs. This trial was registered at clinicaltrials.gov as NCT 00592137.
INTRODUCTION
The prevalence of overweight in American youth has nearly tripled in the past 3 decades to ≈17% (1). Childhood obesity increases the risk of cardiovascular disease, hypertension, and diabetes mellitus in adulthood, all of which are leading causes of death in the United States (2–4). Yet, reported energy intakes in adolescents changed little from the 1970s to the 1990s according to the National Health and Nutrition Examination Survey (5). This might indicate that low energy expenditure rather than excessive energy intake is contributing to the rising obesity, but this assumes accurate energy intake reporting. To study the role of diet in the maintenance of healthy body weight, accurate measures of energy intake are needed, especially in children (6). Underreporting in adults has been associated with income (7–9), education (9, 10), socioeconomic status (11), body image (12), social desirability (12, 13), restrained eating (7), and depression (14). An important factor influencing underreporting is body mass index (BMI; in kg/m2) in both adults and adolescents (15).
Although adolescence represents an active period of growth, the reporting of energy intake during this life stage has not been extensively studied. Livingstone et al (16) showed favorable agreement between weighed diet records and corresponding measures of total energy expenditure (TEE) in 7- and 9-y-old children; however, by adolescence, estimates of energy intake using food records, even when given prior instructions and demonstrations, were significantly lower than measurements of TEE (17). In 118 white and black boys and girls aged 9–19 y, mean daily energy intake was underreported by 17–33% of TEE and tended to increase with age (17). Bandini et al (18) showed that errors incurred in food record keeping appeared to be significantly greater in obese adolescents, even though they were issued detailed instructions on how to estimate food portion size. Similarly, obese sixth graders underreported energy intake to a greater degree than did lean children (19). These studies predate the increase in the prevalence of adolescent overweight in the United States (19), and underreporting may now be greater.
With the increasing and earlier onset of overweight and obesity in adolescents and because dietary habits formed early in life have a considerable effect on long-term health status (20, 21), it is critical to identify factors contributing to underreporting and misreporting of food intake in this population. Thus, the first objective was to compare TEE, measured by using doubly labeled water (DLW), with self-reported energy intake, from diet records collected before 2 controlled feeding periods, in overweight and obese adolescent boys and girls.
In addition to the adolescent studies mentioned above, there are now many adult studies comparing self-reported energy intake with TEEDLW. None of these studies, however, compared TEEDLW with a rigorous metabolic balance method to ensure that there are no errors introduced by assumptions related to metabolizable energy values of foods. A second objective was to compare TEE and metabolizable energy intake for weight stability (MEIwtstb), which was directly measured during 2 controlled metabolic studies under strict dietary control. By collecting diet records, MEI, and TEE in 2 time periods, we were also able to assess the measurement error of these methods.
SUBJECTS AND METHODS
Overweight adolescents, aged 12–15 y, were recruited to participate in two 3-wk metabolic balance sessions, separated by a 3-wk washout period, to study the effect of calcium intake on the predictors of fat balance, ie, fecal fat excretion and lipid oxidation (reported elsewhere). This study design allowed us to obtain duplicate measures of energy intake and expenditure. All subjects resided in a residence hall on the Purdue University campus in a supervised environment for the balance studies, which were conducted at a summer research camp. During the 3-wk washout period, the subjects returned home and resumed their usual activity and diet. During each balance session, subjects consumed an energy-controlled diet that provided their estimated individualized energy need, which was calculated on the basis of their body weight (ranging from 1521 to 3770 kcal/d). Informed consent was given by all subjects and by their guardians before participation in the study. All procedures were approved by the Purdue University Institutional Review Board.
Reported energy intake by food records
Subjects were provided with written instructions to record their food intake over 6 consecutive days before the camp and for 3 d between the 2 sessions of the camp. During the first 3-wk session, a dietitian reviewed the records with the subjects individually. This additional training resulted in no significant difference in energy intake between the precamp and washout diet records, so all 9 records were combined. A standardized form was used to record all foods and fluids consumed. Each subject was issued instructions with illustrations of foods and portion sizes that helped them to estimate the amounts of food eaten at home or outside the home. Subjects were requested to describe everything that they ate and drank and to indicate the time and place of consuming these food items. They were told to record the amounts of food eaten in portion sizes of cups, teaspoons, tablespoons, ounces, slices, or inches. They were also asked to draw a picture to indicate the size of a portion. A dietitian met with the adolescents when they came to camp for the balance study and clarified entries in the diet records. We also counted the total number of foods reported by the subjects as meal components, snacks, candy, dessert, soft drinks, and condiments. The estimated energy intake was calculated by using a computerized database and analysis program (NDS-R software, version 5.0–3.5; the University of Minnesota). The Atwater energy equivalents of 4, 4, and 9 kcal/g of protein, carbohydrate, and fat, respectively, were the standard values used in the database system to convert grams of macronutrient intake to metabolizable energy.
Dietary intake and MEI during balance period
Dietary intake was controlled; 3 meals and 2 snacks were prepared by the staff and consumed by the subjects under the supervision of counselors. A 4-d-cycle menu was used. A sample menu is given in Supplemental Table A under “Supplemental data” in the online issue. All food and beverages were prepared with deionized water and weighed to the nearest 0.1 g. Estimated calcium intake was 650 mg/d in one metabolic session and 1300 mg/d in the other session in randomized order. Otherwise, the basal diet was the same between sessions within individuals. Portions of food between individuals varied to meet energy prescription. The macronutrient content of the diet was 55% energy from carbohydrate, 15% from protein, and 30% from fat. Each subject’s energy intake (EI) requirement was estimated by using formulas in the Dietary Reference Intakes (22). The subjects were assumed to be physically active [physical activity level (PAL) is ≥1.6 but <1.9 times resting energy expenditure (REE)] for estimating their energy requirements for the balance period (22). Basal diets of 5 different energy intake levels (prescribed energy intake) were prepared each day and color coded to avoid confusion. For those individuals whose energy needs were above that of the basal diet, additional energy was provided by cookies of similar macronutrient composition to the basal diet to bring them as close as possible to their predicted energy intake requirement.
Meal composites were prepared daily with the identical specifications as those of the meals. The composites were frozen and at a later date thawed, homogenized, and freeze-dried. Gross energy content was measured by bomb calorimetry (model 1281; Parr Instruments Company, Moline, IL).
The urine and feces of the subjects were collected for the duration of the study period. Urine was collected in acid wash containers and pooled in 24-h collections; the final collection was the first sample of the following morning. Samples were processed and the total volume of urine was determined on a daily basis. Specific gravity (g/mL) of urine was measured using a digital urine refractometer (Misco, Cleveland, OH). The urine was acidified with concentrated hydrochloric acid (1% by volume) and aliquots were frozen at −10°C. Urine samples were combined in a weighted fashion and pooled by sessions 1 and 2 of the metabolic balance study. The urine samples were freeze-dried, pulverized, and stored in Whirlpak bags (Fisher Scientific, Pittsburgh, PA) for later analysis by bomb calorimetry.
Fecal samples were measured in preweighed containers and frozen. The samples were pooled for 24 h, diluted with concentrated hydrochloric acid and ultra-high-purity water, and homogenized with a stomacher (Laboratory-Blender 3500; Tekmar, Cincinnati, OH). The samples were freeze-dried (VirTis; SP Industries, Gardiner, NY) and pulverized and the percentage of fecal solids was calculated. The fecal samples were stored in plastic tubes until further analysis.
Measured MEI unadjusted for body weight (kcal/d; MEIunadj) was calculated as gross energy of the diet − gross energy in urine − gross energy in feces. MEI for weight stability (MEIwtstb) was calculated as MEIunadj − (weight change in kg × 7600 kcal/kg)/number of days. Assuming that adipose tissue is 20% water and that each gram of fat stored has an energy equivalent of 9.5 kcal/g, we used a caloric equivalent of 7.6 kcal for 1 g adipose tissue (21). We assumed that weight loss was primarily fat loss because any shift in water would have occurred during the first-week equilibration before adjusting for body weight, and protein losses usually account for <5% of total weight loss.
Resting energy expenditure
REE was measured by indirect calorimetry (MedGraphics Cardiopulmonary Diagnostics Systems; MedGraphics Corporation, St Paul, MN) in the fasting state during the second week of each balance period. Each subject rested in a recumbent position for ≈35 min with the ventilated hood system in place for the last 15 min. Rates of oxygen consumption and carbon dioxide production were measured and averaged from 15 consecutive 1-min expired air samples. REE was calculated as oxygen consumption rate (L/min) × oxygen associated with the respiratory-exchange ratio of the expired air (kcal/L).
Total energy expenditure
DLW was administered during both sessions of the balance study, a week after arrival. TEE was measured by an established procedure (23). Briefly, subjects were weighed in the morning and baseline urine was collected. An oral dose of 1.8 g/kg total body water of ≈10 AP (atom %) H218O and 0.14 g/kg total body water 99.9 AP D2O (Cambridge Isotope Laboratory, Andover, MA) was administered to the subjects at 0800. To maintain urine production, subjects were given preweighed portions of deionized water over 4–6 h. Postdose spot urine samples of 4.5 mL were taken and stored from voids at 2, 4, and 6 h, and complete urine samples were maintained for balance studies. Urine samples were also collected at the end of week 1 and week 2, and all samples were stored in labeled cryotubes and frozen until analysis.
Analysis
Baseline, 6-h, day 7, and day 14 urine samples were analyzed for deuterium and 18O abundances. Samples were decolorized with dry carbon black. Isotope enrichments of the urine samples were prepared using standard vacuum techniques and analyzed in duplicates by using isotope ratio mass spectrometry (Delta Plus and Delta V; Thermo Scientific, Waltham, MA).
Deuterium was measured by conversion of the water sample to hydrogen gas on chromium at 850°C (24). Oxygen-18 was measured by equilibration with carbon dioxide as adapted to use on the Thermo Scientific Gas Bench system (25). Known gravimetric dilutions of the dose water were analyzed to determine the exact dose. Isotope dilution spaces were calculated by the plateau method (26). Mean daily carbon dioxide production rate (rCO2 in mol/d) was calculated by using equation A6 as modified by Racette et al (26, 27). Energy expenditure by the DLW method was calculated from a modified Weir's equation by use of V̇CO2 from DLW and V̇O2 calculated from the food quotient (28). Analytic precisions were 1 and 0.15 permil for deuterium and oxygen-labeled water, respectively. The analytic variation in DLW measurements was 2.9% (23). Internal quality of each of the DLW analyses was confirmed by using the relative dilution space (deuterium and oxygen-18 between 1.00 and 1.08) and by comparing TEE from the 2 initial and final specimens (<10%).
Calculations
Error in the food record reporting was calculated as the difference between reported intake during the prestudy and TEE during the study measured by DLW. Reporting bias was calculated as [(total energy intake − TEE)/TEE] × 100.
During the trial period, fasting weight was recorded on a daily basis with consistent clothing. The final weight change was calculated as the difference between the weight at the end of the first week of camp (3-d average) and the weight recorded on the last day of the camp (end of the third week; 3-d average). This time coincided with the time that the DLW was administered and analyzed. We used dual-energy X-ray absorptiometry (Prodigy; GE Health Systems–Lunar, Madison, WI) to determine fat mass, the percentage of body fat and fat-free mass in our subjects. Each subject's PAL during the 2 study periods was calculated as TEE/REE.
Statistical analysis
Data in text and tables are presented as means ± SDs. Paired and unpaired t tests were used for group comparisons. Reported energy intake and TEEDLW, MEIwtstb, and TEEDLW were compared in boys and girls by a paired t test. The average TEEDLW of the 2- to 3-wk sessions was used because no differences were shown between the sessions. The average energy intake of the precamp and between-camp session diet records were used. Statistical analyses were performed by analysis of variance using the general linear model (GLM and MIXED procedure) (SAS version 9.1; SAS Institute, Cary, NC). The reproducibility of the methods (% CV) was calculated as the root mean squared error. Relations among variables were identified using linear regression analysis. A stepwise approach was used to include only variables in our statistical model that were statistically significant. Pearson correlation coefficient (r) was used to assess associations between variables. Statistical differences between and within groups were considered significant at P < 0.05.
RESULTS
There were no significant differences in the age and height of the 20 girls and 14 boys who participated in the study (Table 1). Average weight, BMI, and percentage body fat were higher in girls than in boys. All the girls in the study were overweight (BMI >95th percentile). Among the boys, 8 were overweight, 4 were “at risk” of being overweight (BMI: 85–95th percentile), and 2 had a healthy body weight. The latter 2 boys had been classified as overweight at the time of screening.
TABLE 1.
Subject characteristics1
| Girls (n = 20) | Boys (n = 14) | |
| Age (y) | 13.4 ± 0.8 | 13.7 ± 0.7 |
| Height (cm) | 160.9 ± 5.2 | 162.9 ± 6.7 |
| Weight (kg) | 85.8 ± 14.1 | 73.1 ± 13.9a |
| BMI (kg/m2) | 33.0 ± 4.9 | 27.4 ± 4.3a |
| Body fat (%) | 46.0 ± 4.4 | 36.3 ± 10.0a |
| Lean body mass (kg) | 44.3 ± 5.7 | 43.7 ± 7.0 |
All values are means ± SDs. Superscript letters indicate significant differences between sexes, P < 0.05 (Student's t test).
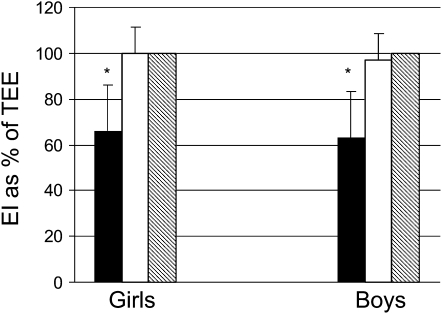
When TEEDLW and MEIwtstb were compared within subjects, there was no significant difference (P > 0.05; Figure 1, Table 2). Also, there was no significant difference between sessions (P > 0.05; Tables B–F under “Supplemental data” in the online supplement). There was a strong correlation between the TEEDLW measures in the 2 sessions of the balance study (r = 0.92, P < 0.0001; % CV = 3.9). MEIwtstb was moderately correlated between sessions (r = 0.50, P < 0.05; % CV = 9.9). Average TEEDLW and MEIwtstb were moderately correlated (r = 0.59, P = 0.0002).
FIGURE 1.
Mean (±SD) reported energy intake from diet records (solid bars), average weight-adjusted metabolizable energy intake (MEIwtstb; open bars), and total energy expenditure (TEE) by doubly labeled water (hatched bars) in overweight boys and girls [energy intake (EI) expressed as % of total energy expenditure measured by doubly labeled water (TEEDLW)]. *Reported energy intake significantly differed from MEI and TEEDLW by paired t test, P < 0.05. Average weight-adjusted MEI was not significantly different from TEEDLW as evaluated by paired t tests (P > 0.05).
TABLE 2.
Energy intake and expenditure during the controlled metabolic periods in overweight adolescents1
| Girls (n = 20) | Boys (n = 14) | |
| TEE (kcal/d) | 2835 ± 336 | 3332 ± 312a |
| REE (kcal/d) | 1450 ± 291 | 1703 ± 301a |
| Physical activity level (TEE/REE) | 2.02 ± 0.41 | 1.99 ± 0.32 |
| Prescribed energy intake2 (kcal/d) | 2111 ± 113 | 3070 ± 277a |
| Gross energy intake3 (kcal/d) | 2283 ± 124 | 3341 ± 317a |
| Gross energy urine (kcal/d) | 26 ± 12 | 25 ± 9 |
| Gross energy feces (kcal/d) | 232 ± 63 | 287 ± 74a |
| MEIunadj (kcal/d) | 2036 ± 140 | 3028 ± 266a |
| MEIwtstb (kcal/d) | 2804 ± 259 | 3225 ± 34a |
All values are means ± SDs. TEE, total energy expenditure (measured by doubly labeled water); REE, resting energy expenditure (measured by indirect calorimetry); MEIunadj, unadjusted metabolizable energy intake (gross energy of food − gross energy of feces − gross energy of urine); MEIwtstb, metabolizable energy intake for weight stability [MEIunadj − (weight change × 7600 kcal/kg)/number of days]. Superscript letters indicate significant differences between sexes, P < 0.05 (Student's t test).
Prescribed energy intake is the energy need calculated for each individual subject (predicted energy intake requirements) according to the estimated energy requirement formulas in the Dietary Reference Intakes report (22).
Gross energy intake is the energy of the food matter consumed by the subjects during the metabolic study period, which was measured by using bomb calorimetry.
TEEDLW was significantly higher in boys than in girls (P < 0.05) (Table 2). Reported energy intake in the precamp period was significantly lower (P < 0.0001) than the TEEDLW during the study period in both boys and girls (Figure 1). No significant sex differences were observed for reported fat, carbohydrate, or protein intake (Table 3). The energy intake in girls was underreported by 945 ± 626 kcal/d and in boys by 1238 ± 633 kcal/d. There was a relative error of 34% for girls and 37% for boys. The combined error in reported intakes from dietary records was ≈1065 ± 636 kcal/d or a relative error of 35 ± 20%. Energy intake represented 66% of TEEDLW in girls and 64% of TEEDLW in boys.
TABLE 3.
Summary of reported energy and macronutrient intakes using food records in overweight adolescents1
| Girls (n = 20) | Boys (n = 14) | |
| TEI (kcal/d) | 1890 ± 702 | 2094 ± 563 |
| TEI:TEE ratio2 | 0.66 ± 0.22 | 0.63 ± 0.18 |
| Fat intake (g/d) | 79 ± 30 | 88 ± 30 |
| Fat intake (kcal/d) | 708 ± 269 | 794 ± 270 |
| Carbohydrate intake (g/d) | 235 ± 97 | 249 ± 74 |
| Carbohydrate intake (kcal/d) | 939 ± 387 | 995 ± 294 |
| Protein intake (g/d) | 64 ± 21 | 79 ± 30 |
| Protein intake (kcal/d) | 255 ± 83 | 317 ± 120 |
All values are means ± SDs. TEI, total energy intake (total energy intake reported using food records); TEE, total energy expenditure (measured by using doubly labeled water). There were no significant differences due to sex (Student's t test).
Ratio between reported TEI from food records and TEE.
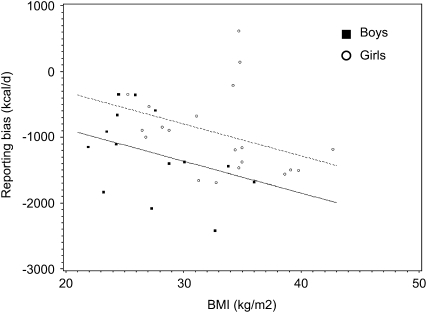
A stepwise regression model that included dietary fat (g), BMI, and sex explained 86.4% of the variance in reporting error. The error in reported intake became more negative (ie, increased reporting bias) with increasing BMI (Figure 2). The partial R2s for dietary fat, BMI, and sex are 0.59, 0.15, and 0.13, respectively. The reported dietary protein and carbohydrate (g or kcal) also positively correlated with underreporting (P < 0.0001). When analyzed separately by sex, the same significant relations existed.
FIGURE 2.
Relation between BMI and underreporting as determined by linear regression (underreporting = BMI, sex, r2 = 0.18, P = 0.05). Girls are represented by circles and the dashed line, and boys are represented by squares and the solid line.
We evaluated a subset of 6 subjects, 4 girls and 2 boys, whose reported energy intake was within ±500 kcal/d TEE. Reported total fat, carbohydrate, and protein were all higher than for other subjects. Five of the 6 subjects were in the 94–98th percentile of BMI for age, which classifies them as “overweight.” All of these subjects had a higher lean body mass (by dual-energy X-ray absorptiometry) compared with the other subjects. These subjects had detailed dietary records with an average of ≥14 food items in their diet records every day, with up to a maximum of 20 items compared with the entire group who reported ≈10 ± 8 items. These 6 subjects reported consuming more snacks, condiments, and desserts than their counterparts.
MEIwtstb, MEI unadj, and prescribed energy intake in boys and girls are shown in Table 2. MEIwtstb was significantly higher in boys than in girls (P < 0.05). When TEEDLW was compared with the MEIwtstb during the balance period, there was a stronger correlation (r = 0.59, P = 0.0002) than for TEEDLW and EI estimated from diet records (r = 0.34, P = 0.049). We observed a higher weight loss in girls than in boys during the balance period. The average weight change in girls was −1.45 ± 0.49 kg in session 1 and −1.38 ± 0.52 kg in session 2 compared with −0.37 ± 0.72 kg in session 1 and −0.38 ± 0.68 kg in session 2 for boys. During the balance period, MEIwtstb represented 99% of the TEE for boys and girls combined. REE was positively correlated with TEE (r = 0.48, P = 0.004). PAL and reporting bias were not correlated (r = 0.13, P = 0.45). Lean body mass was strongly correlated with TEE (r = 0.52, P = 0.002).
DISCUSSION
To understand the role of diet in childhood obesity, accurate assessment tools for assessing energy intake and expenditure are needed. We evaluated the precision of the methods for both energy intake and expenditure assessment. Measurement error was appreciable only for self-reported food records, which led us to explore explanations for reporting bias. Our study shows that energy underreporting bias increases with increasing BMI.
A primary finding of this study is that self-reported energy intake from diet records had a 35% reporting bias in overweight and at-risk adolescents. Body weight was significantly related to reporting bias in both boys and girls, which suggests that recording errors increased with increasing body weight. Energy underreporting bias in adolescents using TEEDLW as the criterion method has been reported previously (18, 29). In one study, Swedish adolescents (mean age: 15 y) using 7-d diet records underestimated energy intake by 18% for boys and 22% for girls, particularly those with a tendency for overweight and increased body weight (29). A study in obese adolescents showed a reporting bias of 42% from diet records in obese adolescents compared with 20% in nonobese adolescents (18). Previous studies did not measure actual MEIwtstb or include replicate measures in children.
Conceivably, the high percentage of underreporting in our study could be due to a difference between energy expenditure during the prestudy periods when food records were collected and the study sessions when TEEDLW was determined. Simultaneous collection of diet records and measurement of TEEDLW during the metabolic studies was not possible because of the controlled energy intake during both sessions. Predicted weight change during the metabolic sessions based on the prestudy diet records was not the same as the observed weight change. The difference of 18% between MEIwtstb and MEI unadj suggests that our subjects consumed 18% less than they actually needed to maintain weight, especially in girls. Calculated PAL using TEEDLW and REE measured during the balance period was ≈2. However, we used a value for physical activity of 1.26 (corresponding to PAL levels of ≥1.6 but <1.9) (22) to estimate the energy requirements for our subjects for the balance period on the basis of the assumption that our subjects were active at home and would be active in camp. The calculated PAL of 2.01 during camp falls into the “very active” category (≥1.9 but <2.5) (22). Nevertheless, our value was similar to that of others who measured TEE and energy intake in the same period (17, 18). With our study design, we were unable to distinguish the cause of the discrepancy between TEE and prescribed energy intake among inaccuracies in the Dietary Reference Intake formulas (22), differences in physical activity, or differences in energy expenditure of our volunteers.
Studies in younger children whose dietary records were entered by a parent or guardian showed good agreement between reported intake and energy expenditure measured by DLW (16, 28). However, studies in older children and adolescents where the child or adolescent had to record their own intake have shown a bias toward underreporting. Livingstone et al reported the results of the dietary assessment (using 7-d-diet weight records) of 78 children and adolescents between 3 and 18 y of age using both weight dietary records and diet histories. Underreporting increased with age, ie, −11%, −22%, and −27% for 12, 15, and 18 y of age, respectively (16). Although Livingstone et al's (16) error was high at the age of our subjects, it was lower than ours, likely due to the use of weighed dietary records.
Postulated reasons behind reporting bias based on different studies include difficulty in estimating portion size (despite written instructions) and failure to recognize ingredients in mixed foods and snack foods in this age group. The recording procedure is regarded as a burden in preschool children (30) and in adolescents (17). The use of weighed diet records as in the study by Livingstone et al (16) would be even more burdensome, but the error was reduced. Subjects in the study by Bandini et al (18) were given detailed instructions on recording dietary information. Yet, the reporting bias in obese adolescents in this study was comparable to the bias observed in our study in which only written instructions were provided with the diet records to assist in estimating portion sizes. These large underreporting errors imply that there is a need to develop new methods in training subjects, especially for obese adolescents, or methods that require less subject burden.
Dietary fat contributed to a higher variance in reporting error as compared with carbohydrates or protein, but there was no significant relation between underreporting and any of the macronutrients (% kcal), which suggests that all macronutrients were underreported by the teens. Other studies have shown underreporting of specific macronutrients (15, 31, 32). Livingstone et al (33) reported that the underreporting of total food intake was explained by a selective underreporting of snacks. The only subjects who reported energy intake accurately in our study had a higher proportion of lean body mass. These subjects better reported snacks, candy, dessert, soft drinks, and condiments, to name a few in their diet records.
In our study, MEIwtstb measured using bomb calorimetry was not different from TEEDLW, and both methods had percentage CV <10%—3.9% for TEE and 9.9% for MEIwtstb (this variation includes analytic and physiologic components). Precision for TEEDLW in adults was 5.1% (23). Thus, the methods can be considered equivalent in accuracy and test-retest precision, but TEEDLW requires considerably less subject and researcher burden. The lack of difference in results between MEIwtstb and TEEDLW is similar to that of a study in adult males and females in which the difference in TEE measured by DLW and MEI by balance was within 0.3%, a nonsignificant difference (34). When predicted MEI and MEIunadj were compared, we showed that predicted MEI overestimated measured MEI by only 3% (P = 0.63). This indicates that the assumed metabolizable energy values of the diets were accurate when averaged across the diets. Our finding is similar to a study by Seale's group (35), who did not find a significant difference between free-living energy expenditure measured by DLW and MEI in 4 adult men.
Strengths of our study included the replicated direct comparison of measured MEI on a controlled diet to TEEDLW in a vulnerable population, ie, obese adolescents, as a reference for evaluating self-reported energy intakes. A limitation of this study was the use of a convenience population, which may not be representative. Another limitation of the study was the necessary displacement in time of self-reported energy intake and measurement of TEEDLW despite an attempt to adjust for changing weight. Diet assessment by diet records is a limitation that we assessed in this study.
Inaccuracy of food intake reporting has important implications for assessing the role of diet in childhood obesity and for evaluating the efficacy of intervention. Limited progress has been made in understanding the determinants and nature of underreporting in the adolescent age group. Our study shows that underreporting bias increases with increasing BMI. This may explain the apparent discrepancy between the increasing prevalence of obesity and the lack of a parallel increase in reported energy intake (5).
Supplementary Material
Acknowledgments
We appreciate the statistical support from Purdue's Statistical Consulting Service. We thank Ania Kempa-Steczko for coordinating the laboratory procedures during the metabolic balance study. We also thank the Camp Calcium staff and the study subjects.
The authors’ responsibilities were as follows—CMW, BRM, DT, and WWC: study design; DAK: diet record instruction; RS and BRM: conducting the study and data collection; YH: analysis of fecal samples (boys) by bomb calorimetry; DAS: doubly labeled water analysis; RS, BRM, and BAC: data analysis. All authors were responsible for preparing the manuscript. None of the authors had a personal or financial conflict of interest.
REFERENCES
- 1.CDC National Center for Health Statistics NHANES data on the prevalence of overweight among children and adolescents: United States, 2003–2006. Available from: http://www.cdc.gov/nchs/products/pubs/pubd/hestats/overweight/overweight_child_03.htm (cited 6 April 2009)
- 2.Hoffmans MD, Kromhout D, Coulander CD. Body mass index at the age of 18 and its effect on 32-year-mortality from coronary heart disease and cancer. J Clin Epidemiol 1989;42:513–20 [DOI] [PubMed] [Google Scholar]
- 3.Holbrook TL, Wingard DL, Barrett-Connor E. Sex-specific vs. unisex body mass indices as predictors of non-insulin dependent diabetes mellitus in older adults. Int J Obes 1990;14:803–7 [PubMed] [Google Scholar]
- 4.Sjostrom L. Morbidity of severely obese subjects. Am J Clin Nutr 1992;55:508S–15S [DOI] [PubMed] [Google Scholar]
- 5.Troiano RP, Briefel RR, Carroll MD, Bialostosky K. Energy and fat intakes of children and adolescents in the United States: data from the National Health and Nutrition Examination Surveys. Am J Clin Nutr 2000;72:1343S–53S [DOI] [PubMed] [Google Scholar]
- 6.Guo SS, Roche AF, Chumlea WC, Gardner JD, Siervogel RM. The predictive value of childhood body mass index values for overweight at age 35years. Am J Clin Nutr 1994;59:810–9 [DOI] [PubMed] [Google Scholar]
- 7.Lafay L, Basdevant A, Charles MA, et al. Determinants and nature of dietary underreporting in a free-living population: the Fleurbaix Laventie Ville Sante (FLVS) Study. Int J Obes Relat Metab Disord 1997;21:567–73 [DOI] [PubMed] [Google Scholar]
- 8.Stallone DD, Brunner EJ, Bingham SA, Marmot MG. Dietary assessment in Whitehall II: the influence of reporting bias on apparent socioeconomic variation in nutrient intakes. Eur J Clin Nutr 1997;51:815–25 [DOI] [PubMed] [Google Scholar]
- 9.Pomerleau J, Ostbye T, Bright-See E. Potential underreporting of energy intake in the Ontario Health Survey and its relationship with nutrient and food intakes. Eur J Epidemiol 1999;15:553–7 [DOI] [PubMed] [Google Scholar]
- 10.Johansson L, Solvoll K, Bjorneboe GE, Drevon CA. Under- and overreporting of energy intake related to weight status and lifestyle in a nationwide sample. Am J Clin Nutr 1998;68:266–74 [DOI] [PubMed] [Google Scholar]
- 11.Price GM, Paul AA, Cole TJ, Wadsworth MEJ. Characteristics of the low energy reporters in a longitudinal national dietary survey. Br J Nutr 1997;77:833–51 [DOI] [PubMed] [Google Scholar]
- 12.Taren DL, Tobar M, Hill A, et al. The association of energy intake bias with psychological scores of women. Eur J Clin Nutr 1999;53:570–8 [DOI] [PubMed] [Google Scholar]
- 13.Hebert JR, Clemow L, Pbert L, Ockene IS, Ockene JK. Social desirability bias in dietary self-report may compromise the validity of dietary intake measures. Int J Epidemiol 1995;24:389–98 [DOI] [PubMed] [Google Scholar]
- 14.Kretsch MJ, Fong AKH, Green MW. Behavioral and body size correlates of energy intake underreporting by obese and normal-weight women. J Am Diet Assoc 1999;99:300–6 [DOI] [PubMed] [Google Scholar]
- 15.Torun B, Davies PS, Livingstone MB, Paolisso M, Sackett R, Spurr GB. Energy requirement and dietary energy recommendations for children and adolescents 1-18 years old. Eur J Clin Nutr 1996;50:S37–81 [PubMed] [Google Scholar]
- 16.Livingstone MBE, Prentice AM, Coward WA, et al. Validation of estimates of energy intake by weighed dietary record and diet history in children and adolescents. Am J Clin Nutr 1992;56:29–35 [DOI] [PubMed] [Google Scholar]
- 17.Champagne CM, Baker NB, Delany JP, Harsha DW, Bray GA. Assessment of energy intake underreporting by doubly labeled water and observations on reported nutrient intakes in children. J Am Diet Assoc 1998;98:426–33 [DOI] [PubMed] [Google Scholar]
- 18.Bandini LG, Schoeller DA, Cyr HN, Dietz WH. Validity of reported energy intake in obese and non-obese adolescents. Am J Clin Nutr 1990;52:421–5 [DOI] [PubMed] [Google Scholar]
- 19.Champagne CM, Delany JP, Harsha DW, Bray GA. Underreporting of energy intake in biracial children is verified by doubly labeled water. J Am Diet Assoc 1996;96:707–9 [DOI] [PubMed] [Google Scholar]
- 20.Blair SN, Horton E, Leon AS, et al. Physical activity, nutrition, and chronic disease. Med Sci Sports Exerc 1996;28:335–49 [DOI] [PubMed] [Google Scholar]
- 21.O'Neil CE, Nicklas TA, Myers L, Johnson CC, Berenson GS. Cardiovascular risk factors and behavior lifestyles of young women: implications from findings of the Bogalusa Heart Study. Am J Med Sci 1997;314:385–95 [DOI] [PubMed] [Google Scholar]
- 22.Food and Nutrition Board, National Academy of Sciences (Institute of Medicine) Dietary Reference Intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids 2002. Washington, DC: The National Academies Press, 2005 [Google Scholar]
- 23.Trabulsi J, Schoeller DA. Evaluation of dietary assessment instruments against doubly labeled water, a biomarker of habitual energy intake. Am J Physiol Endocrinol Metab 2001;281:E891–9 [DOI] [PubMed] [Google Scholar]
- 24.Schoeller DA, Ravussin E, Schutz Y, Acheson KJ, Baertschi P, Jequier E. Energy expenditure by doubly labeled water: validation in humans and proposed calculation. Am J Physiol 1986;250:R823–30 [DOI] [PubMed] [Google Scholar]
- 25.Schoeller DA, Luke AH. Rapid 18O analysis of CO2 samples by continuous-flow isotope ratio mass spectrometry. J Mass Spectrom 1997;32:1332–6 [DOI] [PubMed] [Google Scholar]
- 26.Schoeller DA, Leitch CA, Brown C. Doubly labeled water method: in vivo oxygen and hydrogen isotope fractionation. Am J Physiol 1986;251:R1137–43 [DOI] [PubMed] [Google Scholar]
- 27.Racette SB, Schoeller DA, Luke AH, Shay K, Hnilicka J, Kusner RF. Relative dilution spaces of 2H- and 18O-labeled water in humans. Am J Physiol 1994;267:E585–90 [DOI] [PubMed] [Google Scholar]
- 28.Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol 1949;109:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bratteby L-E, Sandhagen B, Fan H, Enghardt H, Samuelson G. Total energy expenditure and physical activity as assessed by the doubly labeled water method in Swedish adolescents in whom energy intake was underestimated by 7-d diet record. Am J Clin Nutr 1998;67:905–11 [DOI] [PubMed] [Google Scholar]
- 30.Davies PS, Coward WA, Gregory J, White A, Mills A. Total energy expenditure and energy intake in the pre-school child: a comparison. Br J Nutr 1994;72:13–20 [DOI] [PubMed] [Google Scholar]
- 31.Black AE, Prentice AM, Goldberg GR, et al. Measurements of total energy expenditure provide insights into the validity of dietary measurements of energy intake. J Am Diet Assoc 1993;93:572–9 [DOI] [PubMed] [Google Scholar]
- 32.Schoeller DA. Limitations in the assessments of dietary energy intake by self report. Metabolism 1995;44:18–22 [DOI] [PubMed] [Google Scholar]
- 33.Livingstone MBE, Prentice AM, Strain JJ, et al. Accuracy of weighed dietary records in studies of diet and health. BMJ 1990;300:708–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seale JL, Rumpler WV. Comparison of energy expenditure measurements by diet records, energy intake balance, doubly labeled water and room calorimetry. Eur J Clin Nutr 1997;51:856–63 [DOI] [PubMed] [Google Scholar]
- 35.Seale JL, Rumpler WV, Conway JM, Miles CW. Comparison of doubly labeled water, intake balance and direct- and indirect calorimetry method for measuring energy expenditure in adult men. Am J Clin Nutr 1990;52:66–77 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.