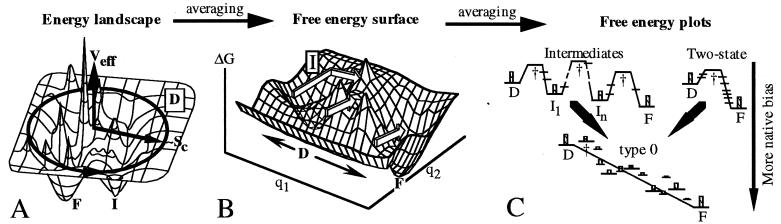
Figure 1.
(A) Protein energy landscape; the radial coordinate correlates with compactness (configurational entropy SC); the angular coordinate symbolizes another >103 coordinates. More compact states generally have lower contact energy EC. The native ensemble F lies near the global minimum; there may be other deep minima I. (B) Thermally averaging the landscape over all but two reaction coordinates removes uninteresting energy spikes (e.g., because of steric hindrance) and leads to a two-dimensional free energy plot. The denatured state D is now a local minimum because of its large entropy. The arrows indicate an intermediate, two-state, and downhill pathway; not all of these necessarily appear simultaneously at constant T. Whether I is an obligatory intermediate or a trap that slows folding depends on its location, relative saddle point heights, etc. (C) Further reduction to one-dimensional pathways. The protein may switch pathways when conditions in B are changed, shifting the location and free energy of minima, maxima, and saddles. Some “nonspecial” states are shown in addition to transition states and intermediates. At weak native bias (e.g., [GuHCl] ≠ 0) a two-state or intermediate scenario results: only D, I, and F are significantly populated during folding (indicated by maximum population histograms). At strong native bias (SC nearly compensated by EC during folding), a type 0 scenario results. The rate is dictated by downhill folding, and I and † join the ranks of the “nonspecial” states, many of which are now populated. Strange kinetics (14) are not caused by thermodynamic barriers, but rather by unproductive diffusive motions “perpendicular” to the one-dimensional reaction coordinate.