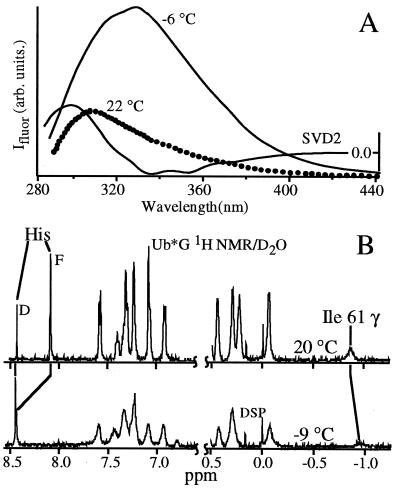
Figure 2.
Representative steady-state cold denaturation measurements. (A) Fluorescence of yeast phosphoglycerate kinase excited at 280 nm; λmax redshifts by over 30 nm on cooling, as evidenced by the second component of a singular value decomposition of the temperature-dependent data. The tryptophan and tyrosine residues become solvent exposed. (B) 1H NMR of Ub*G acquired in D2O with residual water presaturation. The upfield Ile-61 peak is caused by contact with the core tryptophan 45; it disappears in the cold denatured state, indicating core solvation. Both the F and D components of the His-68 peak are resolved; the F peak rapidly decreases in intensity and shifts downfield at lower T. The chemical shift dispersion of the sample decreases toward a random coil.