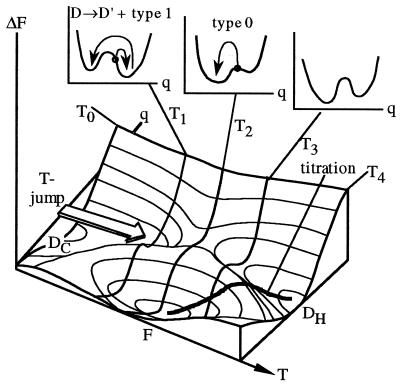
Figure 6.
Free energy as a function of T and reaction coordinate (e.g., protein compactness). At T0, the cold denatured state DC is most stable; at T4, the heat denatured state. At T2 the native state is most stable and at T1 or T3 the equilibrium constant is approximately 1. F is separated from DC and DH by barriers that always lead to cooperative temperature titrations (thick line shows average value of q during titration). A sudden small T-jump from T0 to T1 leaves q constant and the protein in the D well, followed by type 1 activated exponential kinetics (left Inset plot). A larger jump from T0 to T2 leaves the protein closer to the transition state or on a purely downhill type 0 surface if the free energy bias from D to F is too steep (middle Inset plot); the protein folds nonexponentially downhill. A jump from T0 to T3 would presumably result in activated kinetics again because of the onset of heat denaturation.