Abstract
Background
Recent postmortem studies have demonstrated pathological changes, including Purkinje cell loss, in the cerebellum in essential tremor (ET). Toxic exposures that compromise cerebellar tissue could lower the threshold for developing ET. Ethanol is a well-established cerebellar toxin, resulting in Purkinje cell loss.
Objective
To test whether higher baseline ethanol consumption is a risk factor for the subsequent development of incident ET.
Methods
Lifetime ethanol consumption was assessed at baseline (1994-1995) in a prospective, population-based study in central Spain of 3,285 elderly participants, 76 of whom developed incident ET by follow-up (1997-1998).
Results
In a Cox proportional hazards model adjusting for cigarette pack-years, depressive symptoms and community, the baseline number of drink-years was marginally associated with higher risk of incident ET (relative risk, RR = 1.003, p = 0.059). In an adjusted Cox model, highest baseline drink-year quartile doubled the risk of incident ET (RR = 2.29, p = 0.018) while other quartiles were associated with more modest elevations in risk (RR3rd quartile = 1.82 [p = 0.10], RR2nd quartile = 1.75 [p = 0.10], RR1st quartile = 1.43 [p = 0.34] vs. non-drinkers [RR = 1.00]). With each higher drink-year quartile, risk of incident ET increased an average of 23% (p = 0.01, test for trend).
Conclusions
Higher levels of chronic ethanol consumption increased the risk of developing ET. Ethanol is often used for symptomatic relief; studies should explore whether higher consumption levels are a continued source of underlying cerebellar neurotoxicity in patients who already manifest this disease.
Keywords: essential tremor, epidemiology, clinical, cerebellum, ethanol, disease mechanisms, pathogenesis
Introduction
Essential tremor (ET) is one of the most common neurological disorders.1 The underlying disease mechanisms are not clear; however, evidence from clinical and neuroimaging studies implicates a disorder of the cerebellum.2, 3 Recent postmortem studies demonstrate pathological changes in the ET cerebellum, including swellings of Purkinje cell axons and Purkinje cell loss.4-6 With this understanding, one would expect that exposures that compromise cerebellar tissue might predispose to ET or lower the threshold for developing ET. Ethanol is a well-established cerebellar toxin.7-9 We hypothesized that higher baseline ethanol consumption would be a risk factor for the subsequent development of incident ET.
Methods
The Neurological Disorders in Central Spain (NEDICES) is a population-based survey of major age-associated conditions of the elderly (≥65 years) from three communities in Spain (Las Margaritas, Lista and Arévalo).10 Detailed accounts of the study population and survey methods have been reported.10-12 Signed informed consent was obtained upon enrollment.
As documented,10 the baseline in-person evaluation (1994 - 1995) included a questionnaire (demographic, medical, lifestyle information). Data on smoking were collected; prior results suggest that smoking may decrease the risk of incident ET.12 Hence, a priori, cigarette pack-years were a major potential confounder of interest. A general neurologic examination included assessments of action tremor and the motor portion of the Unified Parkinson’s Disease Rating Scale (UPDRS).13 Diagnostic criteria for ET have been described.10
As detailed elsewhere, the follow-up evaluation (1997 - 1998) was similar to the baseline evaluation and the diagnostic approach to ET was identical.10
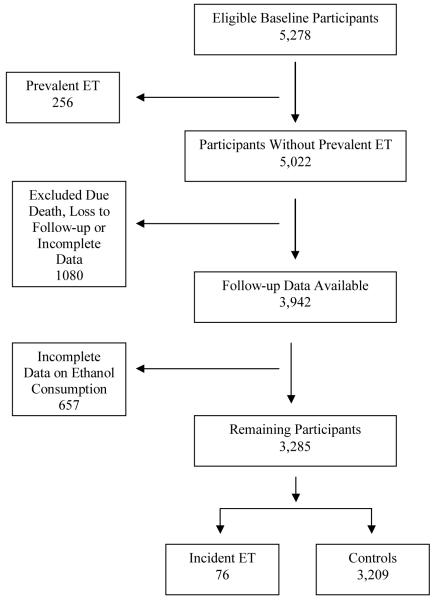
Follow-up data were available on 3,942 of 5,278 baseline participants (Figure). We excluded 657 participants with incomplete data on ethanol consumption, leaving 3,285 participants. These remaining 3,285 participants were similar to the original 5,278 participants in mean (± SD) baseline age (73.5 ± 6.6 vs. 74.3 ± 7.0 years), gender (56.8% vs. 57.3% women) and education (12.7% vs. 12.1% illiterate).
Figure 1. Flow chart from the Neurological Disorders in Central Spain (NEDICES) Study.
There were 76 incident ET cases, including 40 who had in-person baseline examinations and did not have ET on those examinations and 36 who did not have in-person baseline examinations. Baseline handwriting samples of these 36 were reviewed by a senior neurologist (E.D.L.) and none had tremor that was in the ET range (all had Bain and Findley14 handwriting tremor scores ≥1, which are within the range of normal).15 Furthermore, each of the 36 was re-interviewed during the follow-up evaluation to establish that the onset of their tremor had been after the baseline assessment.
In statistical analyses (SPSS version 16.0, Chicago, IL), baseline pack-years = packs of cigarettes smoked/day x years smoked. One drink-year = one drink of ethanol (one 360 mL can of beer or one 120 mL glass of wine or 30 mL of liquor) per day/year. We used Cox proportional hazards models to estimate the relative risk (RR) of incident ET, beginning with an unadjusted model and then included covariates associated with both baseline smoking and incident ET in univariate analyses.
Results
The 3,285 participants included 1,838 (56.0%) who were ethanol drinkers (>0 drink-years) at baseline. These differed from non-drinkers in several respects (e.g., age, Table 1). Mean (SD) follow-up was 3.3 ± 0.8 years (range = 0.1 - 6.6 years). The 76 incident ET cases differed from 3,209 controls by baseline depressive symptoms and cigarette pack-years (Table 1).
Table 1.
Baseline characteristics (ethanol drinkers vs. non-drinkers and incident ET cases vs. control subjects)
| Baseline characteristics | Ethanol Drinkers n = 1,838 |
Non-drinkers n = 1,447 |
Incident ET cases n = 76 |
Control subjects n = 3,209 |
|---|---|---|---|---|
| Age in years (mean ± SD) | 73.1 ± 6.5*** | 74.1 ± 6.7 | 73.7 ± 6.4 | 73.5 ± 6.6 |
| Gender (women) | 648 (35.3%)*** | 1,219 (84.2%) | 45 (59.2%) | 1,822 (56.8%) |
| Educational level Illiterate Can read and write Primary studies Secondary studies |
159 (8.7%)*** 823 (44.8%) 519 (28.2%) 337 (18.3%) |
257 (17.8%) 566 (39.1%) 455 (31.4%) 169 (11.7%) |
12 (15.8%) 35 (46.1%) 18 (23.7%) 11 (14.5%) |
404 (12.6%) 1,354 (42.2%) 956 (29.8%) 495 (15.4%) |
| Cigarette Smoker Current Past Never |
341 (18.6%)*** 773 (42.1%) 723 (39.4%) |
53 (3.7%) 141 (9.7%) 1,253 (86.6%) |
5 (6.6%) 21 (27.6%) 50 (65.8%) |
389 (12.1%) 893 (27.8%) 1,926 (60.0%) |
| Cigarette Pack-years | 24.3 ± 32.0*** | 5.0 ± 18.0 | 9.3 ± 17.8** | 16.0 ± 28.6 |
| Depressive symptoms (Screening question “Do you suffer from depression?”) | 417 (22.7%)*** | 428 (29.7%) | 28 (36.8%)* | 817 (25.5%) |
| Use of an antidepressant medication | 27 (1.5%)** | 41 (2.8%) | 3 (3.9%) | 65 (2.0%) |
| Community Las Margaritas Lista Arévalo |
622 (33.8%)*** 726 (39.5%) 490 (26.7%) |
497 (34.3%) 369 (25.5%) 581 (40.2%) |
38 (50.0%)** 14 (18.4%) 24 (31.6%) |
1,081 (33.7%) 1,081 (33.7%) 1,047 (32.6%) |
| Baseline Drink-years mean ± SD, (median) | 61.9 ± 70.6 (26.3)a | 56.7 ± 71.0 (16.8) | ||
| Baseline Drink-years by smoking strata Non-smokers Cigarette pack-year tertile 1 Cigarette pack-year tertile 2 Cigarette pack-year tertile 3 |
34.5 ± 51.1 (0.0) 94.9 ± 49.2 (98.0) 112.0 ± 93.2 (114.0) 175.5 ± 43.2 (156.0)*a |
31.7 ± 55.8 (0.0) 80.4 ± 69.9 (78.0) 91.8 ± 73.0 (104.0) 110.0 ± 77.9 (116.0) |
p< 0.05
p < 0.01
p < 0.001 (ethanol drinkers vs. non-drinkers; incident ET cases vs. control subjects).
Mann Whitney test
Median number of baseline drink-years was 57% higher (26.3 vs. 16.8) in incident ET cases vs. controls, although this difference did not achieve statistical significance (Table 1). In analyses that stratified by cigarette pack-year tertile, the number of drink-years within each stratum was higher in incident ET cases than controls, although this difference only reached significance in the highest cigarette pack-year tertile (p = 0.035, Table 1).
In an unadjusted Cox proportional hazards model, baseline number of drink-years was not associated with risk of incident ET (RR = 1.001, 95% CI = 0.998 - 1.004, p = 0.52). There were a number of obvious confounding factors (esp. cigarette smoking). In a Cox model that adjusted for cigarette pack-years, depressive symptoms and community, baseline number of drink-years were marginally associated with higher risk of incident ET (RR = 1.003, 95% CI = 1.000 - 1.006, p = 0.059, i.e., for every 10 unit increase in drink years, the risk of ET increased 3%). Adding an additional covariate, use of an antidepressant medication, to the model did not change the results (RR = 1.003, 95% CI = 1.000 - 1.006, p = 0.055).
In a Cox proportional hazards model that adjusted for cigarette pack-years, depressive symptoms and community, highest baseline drink-year quartile was associated with a doubling of risk of incident ET (RR = 2.29, 95% CI = 1.15 - 4.54, p = 0.018, Table 2). With each advancing quartile, the risk of incident ET increased (Table 2), and a test for trend indicated that with each higher drink-year quartile, the risk of incident ET increased on average by 23% (RR = 1.23, 95% CI = 1.05 - 1.43, p = 0.01).
Table 2.
Risk of ET by baseline drink-year quartile
| Baseline Drink-Year Quartile | Unadjusted Model* RR (95% CI), p value | Adjusted Model** RR (95% CI), p value |
|---|---|---|
| Non-drinkers | Reference | Reference |
| Lowest quartile (≤40.7) | 1.13 (0.55 - 2.33), p = 0.73 | 1.43 (0.68 - 2.98), p = 0.34 |
| 2nd quartile (40.8 - 110.0) | 1.31 (0.68 - 2.52), p = 0.42 | 1.75 (0.89 - 3.42), p = 0.10 |
| 3rd quartile (110.1 - 138.0) | 1.27 (0.63 - 2.54), p = 0.50 | 1.82 (0.89 - 3.74), p = 0.10 |
| Highest quartile (>138.0) | 1.49 (0.77 - 2.86), p = 0.24 | 2.29 (1.15 - 4.54), p = 0.018 |
The reference category was non-drinkers.
Unadjusted Cox proportional hazards model.
Cox proportional hazards model adjusting for cigarette pack-years, depressive symptoms and community
In analyses stratified by gender, comparing highest baseline drink-year quartile to non-drinkers, adjusted RRmen = 2.93 (95% CI = 0.79 - 10.81, p = 0.11) and adjusted RRwomen = 1.66 (95% CI = 0.50 - 5.51, p = 0.41).
We created two education strata (high education = primary or secondary studies vs. low education = can read or write or illiterate). In analyses stratified by education, comparing highest baseline drink-year quartile to non-drinkers, adjusted RRhigh education = 2.19 (95% CI = 0.81 - 5.94, p = 0.13) and adjusted RRlow education = 2.25 (95% CI = 0.86 - 5.85, p = 0.097).
In an analysis of daily number of drinks at baseline (rather than drink-years), when those in the highest category (>5 drinks/day) were compared to non-drinkers, RR = 1.94 (95% CI = 0.26 - 14.67, p = 0.52). When those who had 3 - 5 drinks/day were compared to non-drinkers, RR = 2.19 (95% CI = 0.66 - 7.31, p = 0.20). When those who had 1 - 2 drinks/day were compared to non-drinkers, RR = 1.30 (95% CI = 0.78 - 2.17, p = 0.31).
ET cases included 40 who had had an in-person baseline examination and 36 who did not. When we included in the adjusted Cox proportional hazards analysis a covariate indicating the type of baseline evaluation, results did not change (RR = 2.29, 95% CI = 1.15 - 4.54, p = 0.018).
Discussion
The highest quartile of baseline ethanol consumption was associated with substantially higher risk of developing incident ET while other quartiles were associated with more modest elevations in risk. There was a dose-response effect; with each advancing higher drink-year quartile, the risk of developing incident ET increased an average of 23%.
One interpretation of these data is that ethanol, a known cerebellar toxin, lowers the threshold for developing ET, which seems to be a disorder involving the cerebellum. Ethanol is a well-established cerebellar toxin and a variety of noxious changes have been demonstrated including reductions in Purkinje cell number and density, regression of Purkinje cell dendritic arbors, and loss of synapses.7-9, 16 Although the effects are most pronounced in the cerebellar vermis, they are not restricted to that cerebellar region, and gross atrophy of cerebellar hemispheres has been demonstrated in many studies.17, 18 The long term intake of moderate doses of daily ethanol in humans has been shown to result in significant Purkinje cell loss.19 Recent postmortem studies of ET have indicated that there are cerebellar changes including Purkinje cell axonal swellings, Purkinje cell loss, and possibly gliosis in excess of that seen in age-matched control brains.4-6 In some brains, more extensive cerebellar degeneration is evident, including changes in the dendate nucleus.20 A two-hit model has been proposed for a variety of diseases of the central nervous system, with the basic concept being that an initial exposure/injury to a distinct neuronal population can produce permanent and cumulative neurotoxicity and enhance vulnerability to a second set of insults involving that same neuronal population.21-24
One possibility is that the 76 NEDICES participants who developed incident ET between the baseline and follow-up evaluations had pre-clinical ET at baseline (i.e., enough tremor for them to notice but not enough for a diagnosis of ET) and they were self-medicating with ethanol (i.e., they were drinking more ethanol in order to lessen this tremor). We think this is unlikely. First, when asked at baseline whether their arms or legs shook, they answered “no”, indicating that they did not notice tremor. Second, each of the 76 had an in-person neurological assessment or a detailed assessment of handwriting and each was found to have negligible if any tremor. Finally, the assessment of baseline handwriting samples in 36 incident ET cases indicated that a baseline tremor of 1 rather than 0 was not associated with more ethanol consumption (χ2 = 10.27 [p = 0.25] for baseline handwriting tremor score vs. baseline drink-year quartile), indicating that even this negligible amount of tremor did not have as a correlate greater consumption of ethanol.
NEDICES participants in the highest quartile of ethanol consumption were consuming on average 3 - 4 standard drinks per day; this amount is consistent with prior clinical definitions of heavy ethanol consumption (≥3 standard drinks/day in women and ≥4 in men)25 and commensurate with chronic ethanol intake levels shown to be associated with Purkinje cell degeneration in humans.19
This study had limitations. The findings may be generalized only to ET cases age ≥65 years. Also, our mean follow-up time was modest and longer follow-up would likely have resulted in more incident ET cases and greater study power.
Our findings have possible clinical implications. We observed that the highest levels of chronic ethanol consumption seemed to increase the risk of developing ET. The mechanism, though not established, could involve cumulative cerebellar neurotoxicity. By implication, it is conceivable that chronic ethanol use, while symptomatically beneficial for patients, could be a continued source of underlying cerebellar neurotoxicity and, therefore, be a continued contributor to the progression of the disease. If this were the case, then patients would need to be cautioned about the potential deleterious effects of high levels of ethanol on their underlying disease. Future studies should explore these issues.
Acknowledgments and Funding
The authors gratefully acknowledge the help of the other members of the NEDICES Study Group: S. Vega, J.M. Morales, R. Gabriel, A. Portera-Sánchez, A. Berbel, A. Martínez-Salio, J. Díaz-Guzmán, J. Olazarán, J. Pardo, J. Porta-Etessam, F. Pérez del Molino, J. Rivera-Navarro, M. Alonso, C. Gómez, C. Saiz, G. Fernández, P. Rodríguez and F. Sánchez-Sánchez. Finally, we also wish to express our sincere thanks to J. de Pedro-Cuesta, M.J. Medrano, and J. Almazán, and the municipal authorities, family doctors, nurses, and the populations of Getafe, Lista, and Arévalo county. NEDICES was supported by the Spanish Health Research Agency and the Spanish Office of Science and Technology. Dr. Louis is supported by NIH R01 NS042859, R01 NS039422, and ES P03 09089 from the National Institutes of Health, Bethesda, MD.
The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence (or non-exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd and its Licensees to permit this article (if accepted) to be published in Journal of Neurology, Neurosurgery & Psychiatry and any other BMJPGL products to exploit all subsidiary rights, as set out in our licence (http://jnnp.bmj.com/ifora/licence.pdf).
Other members of the Neurological Disorders in Central Spain (NEDICES) Study Group are listed in the Acknowledgments.
Footnotes
Disclosures: The authors report no conflicts of interest.
Statistical Analyses: The statistical analyses were conducted by Dr. Louis.
References
- 1.Louis ED, Ottman R, Hauser WA. How common is the most common adult movement disorder? estimates of the prevalence of essential tremor throughout the world. Mov Disord. 1998;13(1):5–10. doi: 10.1002/mds.870130105. [DOI] [PubMed] [Google Scholar]
- 2.Leegwater-Kim J, Louis ED, Pullman SL, et al. Intention tremor of the head in patients with essential tremor. Mov Disord. 2006;21(11):2001–2005. doi: 10.1002/mds.21079. [DOI] [PubMed] [Google Scholar]
- 3.Louis ED, Shungu DC, Chan S, Mao X, Jurewicz EC, Watner D. Metabolic abnormality in the cerebellum in patients with essential tremor: a proton magnetic resonance spectroscopic imaging study. Neurosci Lett. 2002;333(1):17–20. doi: 10.1016/s0304-3940(02)00966-7. [DOI] [PubMed] [Google Scholar]
- 4.Axelrad JE, Louis ED, Honig LS, et al. Reduced purkinje cell number in essential tremor: a postmortem study. Arch Neurol. 2008;65(1):101–107. doi: 10.1001/archneurol.2007.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shill HA, Adler CH, Sabbagh MN, et al. Pathologic findings in prospectively ascertained essential tremor subjects. Neurology. 2008;70(16 Pt 2):1452–1455. doi: 10.1212/01.wnl.0000310425.76205.02. [DOI] [PubMed] [Google Scholar]
- 6.Louis ED, Faust PL, Vonsattel JP, et al. Neuropathological changes in essential tremor: 33 cases compared with 21 controls. Brain. 2007;130(Pt 12):3297–3307. doi: 10.1093/brain/awm266. [DOI] [PubMed] [Google Scholar]
- 7.Ramadoss J, Lunde ER, Chen WJ, West JR, Cudd TA. Temporal vulnerability of fetal cerebellar Purkinje cells to chronic binge alcohol exposure: ovine model. Alcohol Clin Exp Res. 2007;31(10):1738–1745. doi: 10.1111/j.1530-0277.2007.00477.x. [DOI] [PubMed] [Google Scholar]
- 8.Dlugos CA. Ethanol-related increases in degenerating bodies in the Purkinje neuron dendrites of aging rats. Brain Res. 2008;1221:98–107. doi: 10.1016/j.brainres.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andersen BB. Reduction of Purkinje cell volume in cerebellum of alcoholics. Brain Res. 2004;1007(12):10–18. doi: 10.1016/j.brainres.2004.01.058. [DOI] [PubMed] [Google Scholar]
- 10.Benito-Leon J, Bermejo-Pareja F, Louis ED. Incidence of essential tremor in three elderly populations of central Spain. Neurology. 2005;64(10):1721–1725. doi: 10.1212/01.WNL.0000161852.70374.01. [DOI] [PubMed] [Google Scholar]
- 11.Morales JM, Bermejo FP, Benito-Leon J, et al. Methods and demographic findings of the baseline survey of the NEDICES cohort: a door-to-door survey of neurological disorders in three communities from Central Spain. Public Health. 2004;118(6):426–433. doi: 10.1016/j.puhe.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 12.Louis ED, Benito-Leon J, Bermejo-Pareja F. Population-based prospective study of cigarette smoking and risk of incident essential tremor. Neurology. 2008;70(19):1682–1687. doi: 10.1212/01.wnl.0000311271.42596.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fahn SER. Members of the UPDRS Development Committee. In: Fahn SMC, Goldtein M, Calne DB, editors. Recent Developments in Parkinson’s Disease. Macmillan Health Care Information; Florham Park, NJ: 1987. pp. 153–163. [Google Scholar]
- 14.Bain P, Findley LJ. Assessing Tremor Severity. Smith-Gordon; London: 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bain PG, Findley LJ, Thompson PD, et al. A study of hereditary essential tremor. Brain. 1994;117(Pt 4):805–824. doi: 10.1093/brain/117.4.805. [DOI] [PubMed] [Google Scholar]
- 16.Pentney RJ. Measurements of dendritic path lengths provide evidence that ethanol-induced lengthening of terminal dendritic segments may result from dendritic regression. Alcohol Alcohol. 1995;30(1):87–96. [PubMed] [Google Scholar]
- 17.Yokota O, Tsuchiya K, Terada S, et al. Alcoholic cerebellar degeneration: a clinicopathological study of six Japanese autopsy cases and proposed potential progression pattern in the cerebellar lesion. Neuropathology. 2007;27(2):99–113. doi: 10.1111/j.1440-1789.2006.00748.x. [DOI] [PubMed] [Google Scholar]
- 18.Victor M, Adams RD, Mancall EL. A restricted form of cerebellar degeneration occurring in alcoholic patients. Arch Neurol. 1959;1:579–688. [PubMed] [Google Scholar]
- 19.Karhunen PJ, Erkinjuntti T, Laippala P. Moderate alcohol consumption and loss of cerebellar Purkinje cells. BMJ. 1994;308(6945):1663–1667. doi: 10.1136/bmj.308.6945.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Louis ED, Vonsattel JP, Honig LS, et al. Essential tremor associated with pathologic changes in the cerebellum. Arch Neurol. 2006;63(8):1189–1193. doi: 10.1001/archneur.63.8.1189. [DOI] [PubMed] [Google Scholar]
- 21.Serbanescu I, Cortez MA, McKerlie C, Snead OC., 3rd Refractory atypical absence seizures in rat: a two hit model. Epilepsy Res. 2004;62(1):53–63. doi: 10.1016/j.eplepsyres.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Jozwiak J, Jozwiak S, Wlodarski P. Possible mechanisms of disease development in tuberous sclerosis. Lancet Oncol. 2008;9(1):73–79. doi: 10.1016/S1470-2045(07)70411-4. [DOI] [PubMed] [Google Scholar]
- 23.Devlin B, Klei L, Myles-Worsley M, et al. Genetic liability to schizophrenia in Oceanic Palau: a search in the affected and maternal generation. Hum Genet. 2007;121(6):675–684. doi: 10.1007/s00439-007-0358-7. [DOI] [PubMed] [Google Scholar]
- 24.Bradley WG, Jr., Bahl G, Alksne JF. Idiopathic normal pressure hydrocephalus may be a “two hit” disease: benign external hydrocephalus in infancy followed by deep white matter ischemia in late adulthood. J Magn Reson Imaging. 2006;24(4):747–755. doi: 10.1002/jmri.20684. [DOI] [PubMed] [Google Scholar]
- 25.Harasymiw JW, Bean P. Identification of heavy drinkers by using the early detection of alcohol consumption score. Alcohol Clin Exp Res. 2001;25(2):228–235. [PubMed] [Google Scholar]