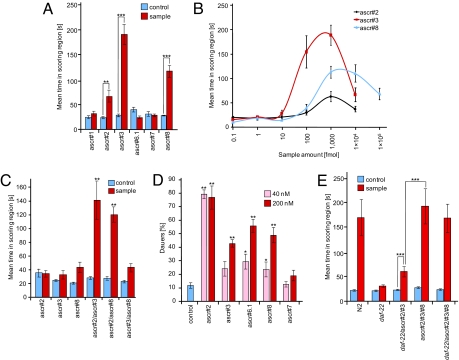
Fig. 4.
Biological evaluation of daf-22-dependent ascarosides identified via DANS. (A) Differential activity of the identified ascarosides in the male attraction assay. ascr#3 showed maximal activity followed by ascr#8 and ascr#2. All other ascarosides did not exhibit significant activity. All compounds were assayed in amounts of 1 pmol. (B) Concentration dependence of male attraction for ascr#2, ascr#3, and ascr#8. ascr#8 shows a broader range of activity than ascr#2 and ascr#3. Data for ascr#2 and ascr#3 have been published (8). (C) Synergistic interactions between the three most active ascarosides. Combinations of ascr#2 and ascr#3 as well as ascr#2 and ascr#8 displayed strong synergy, whereas ascr#3 and ascr#8 did not. ascr#2 and ascr#8 were tested at 100 fmol and ascr#3 at 10 fmol (8). (D) Dauer induction of the different ascarosides. All compounds were assayed at concentrations of 40 nM and 200 nM, and each compound was tested on at least 5 different days. ascr#2 was the most potent dauer inducer and showed significant dauer induction at both concentrations tested. ascr#3, ascr#6.1, and ascr#8 show significant dauer induction only at 200 nM, whereas ascr#7 does not show significant dauer formation at both of the concentrations tested. (E) Reconstitution of male attraction activity of wild-type metabolite extract by combining synthetic ascarosides. A mixture of 20 fmol of ascr#2 and 20 fmol of ascr#3 resulted in significant male attraction, but was less attractive than wild-type metabolite extract containing similar amounts of ascr#2 and ascr#3. However, a ternary mixture of 20 fmol of each ascr#2, ascr#3, and ascr#8 was as active as wild-type metabolite extract. Adding daf-22 metabolite extract does not further increase activity of this ternary mixture. For each data point in Fig. 4 A–C and E, n ≥ 30 animals were used. Error bars, SEM; *, P < 0.01; **, P < 0.001; ***, P < 0.0001, unpaired test with Welch's correction (A and E) and one-factor ANOVA with Tukey–Kramer post test (C).