Figure 1.
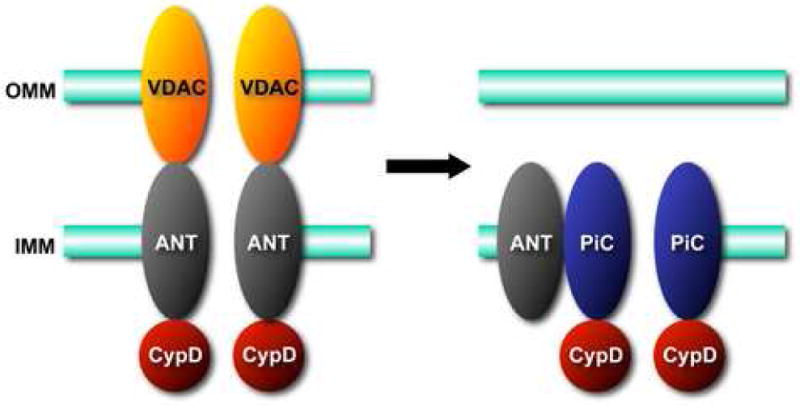
Molecular models for the mitochondrial permeability transition (MPT) pore. A. The original model for the MPT pore, consisting of the voltage-dependent anion channel (VDAC) in the outer mitochondrial membrane (OMM), the adenine nucleotide translocase (ANT) in the inner mitochondrial membrane (IMM), and cyclophilin-D (CypD) in the matrix. B. Revised models in light of recent findings in gene-targeted mice. VDAC is no longer part of the model and it appears that an outer membrane component may not even be necessary for this process. ANT now appears to be more of a regulatory protein, and only CypD remains as an established component. In contrast, the mitochondrial phosphate carrier (PiC) has been added to model as a potential candidate for the pore-unit of the MPT pore.
