Table 2.
Amidine Synthesis Using Primary Amines.a
| entry | primary amines | 4-pentenyl-amidines | yield [%]b | |
|---|---|---|---|---|
| 1 | 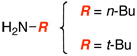 |
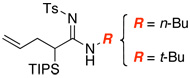 |
9 | ≥95 |
| 2 | 10 | 90 | ||
| 3 | 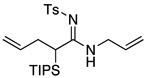 |
11 | 73 | |
| 4 | 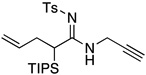 |
12 | 76 | |
| 5 | 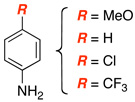 |
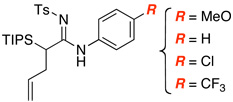 |
13 | 67 |
| 6 | 14 | 85c | ||
| 7 | 15 | 78d | ||
| 8 | 16 | 54 | ||
| 9 | 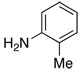 |
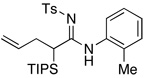 |
17 | 52 |
All reactions utilized ynamide 6, 5.0 mol % Pd(PPh3)4, 1.0 equiv K2CO3, 3.0 equiv RNH2, THF [conc = 0.05 M], 65 °C, 5–8 h.
Isolated yields.
1.0 equiv of amine was used.
Reaction time was 24 h.
