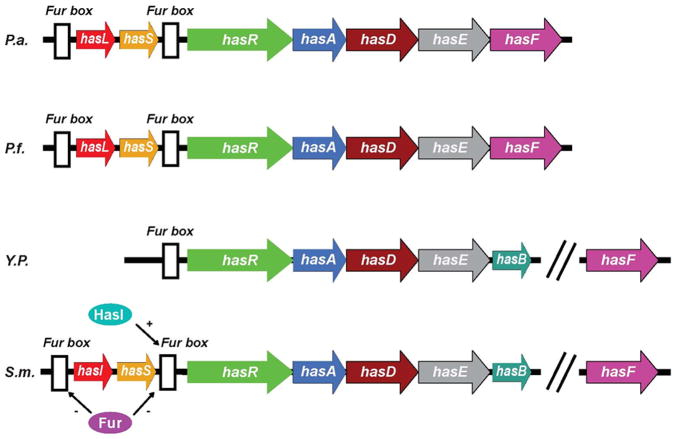
Figure 1.
Genetic organization of the has systems in different bacteria. P.a., Pseudomonas aeruginosa; P.f., Pseudomonas fluorescens; Y.p., Yersinia pestis; S.m., Serratia marcescens. HasI and hasS encode sigma and antisigma factors, respectively. The white boxes indicate consensus Fur boxes. The has operon is directly controlled by Fur (Ferric uptake regulator) [26] and positively regulated by sigma and antisigma factors [158]. HasA is secreted by an ABC transporter consisting of three envelope proteins: HasD, HasE and HasF. HasD, an inner membrane ATPase (the ABC protein) provides the energy for the substrate export; HasE is another inner membrane component and also a membrane fusion protein; HasF, an outer membrane component, is homologous to TolC, which creates a channel in ~ 30Å diameter through the periplasmic space and the outer membrane through which the secreted protein is most likely translocated [159]. Like most proteins secreted by an ABC exporter, hemophores have an α-helical C-terminal secretion signal which can be accessed by SecB, a cytoplasmic chaperone required for hemophore secretion [160]. The secretion signal is recognized by the ABC protein HasD, through modulating its ATPase activity, a HasA-HasDEF multi-protein complex will be formed [161]. Once translocated into the extracellular medium, HasA will bind heme and return it to the HasR receptor. Apo-HasA will be released into the extracellular medium through interaction with TonB-dependent outer membrane receptor.