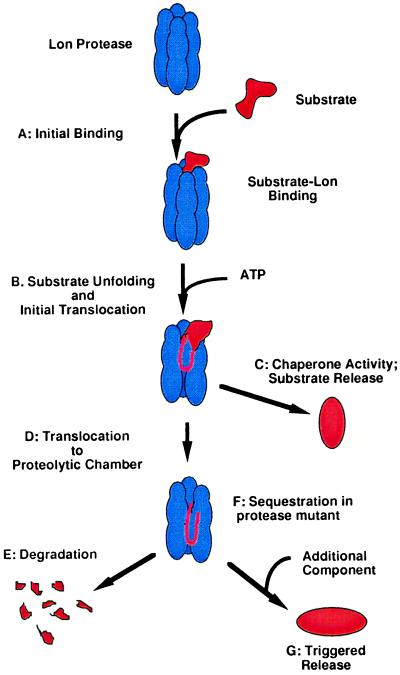
Figure 6.
Model for alternative activities of ATP-dependent proteases and related proteins. After initial recognition and binding of a substrate (A), an energy-dependent step is required for unfolding and translocation of substrates through the ATPase domain (B). In the case of ATPases devoid of a protease domain such as the Clp ATPases, or when degradation is otherwise bypassed, translocation may be followed by release of the protein; in this case the ATPase functions as a chaperone, allowing refolding or remodeling of substrates (C). When a protease-competent domain is present, translocation usually will result in rapid and processive degradation followed by release of products (D and E). If the proteolytic site is inactive, as in the Lon mutants described here or inactivated ClpP (48), or possibly when the protease domain is naturally absent (see Discussion), sequestration of the substrate may occur (F). We propose that in some cases, a triggered release of sequestered substrate would provide a way of delivering an unfolded substrate protein to a new environment (G).