Abstract
Patients treated with teriparatide after prior and ongoing alendronate therapy experience spine BMD increases; however, some continue to be at high risk for fracture, based on persistently low BMD and/or fracture history. The objective of this study was to determine whether a second discrete retreatment course with teriparatide could produce similar biochemical and BMD changes as seen during the first teriparatide course. In the original treatment study, 126 women on alendronate for ≥1 yr were randomized to continue alendronate and receive daily teriparatide, cyclic teriparatide (3-mo cycles), or alendronate alone for 15 mo. Of the 72 patients who completed either original teriparatide regimen, 49 completed a 12-mo follow-up on continued alendronate alone. At that time, 32 patients, who remained at high risk of future fracture, were recruited into the retreatment protocol and 27 completed another course of teriparatide administered daily for 15 mo (including 15 from the original daily treatment group and 12 from the original cyclic treatment group). Bone formation indices (propeptide of type I procollagen and osteocalcin) increased during both teriparatide courses with median 3-mo increments of 120% and 72% above baseline during the original course and 60% and 40% above baseline during retreatment, respectively. Mean spine BMD increments were 6.2% after the first daily course and 4.7% after retreatment and 4.1% after the first course of cyclic teriparatide and 4.9% after retreatment. We conclude that retreatment with teriparatide stimulates bone formation and increases spine BMD to a similar extent as seen during the original teriparatide course. Retreatment with teriparatide may be a viable option for some patients with severe osteoporosis who have received prior teriparatide therapy.
Key words: teriparatide, retreatment, combination therapy, sequential therapy
INTRODUCTION
The FDA currently recommends that the duration of teriparatide (TPTD) therapy for women with osteoporosis be limited to 24 mo,(1) based largely on the findings of a rat carcinogenicity study.(2,3) However, beyond potential safety concerns, shorter treatment periods might also be more efficient with regard to anabolic effect. Prior studies indicated that biochemical measures of bone formation exceed those of bone resorption during the first 3–6 mo of therapy, providing the greatest opportunity for bone growth (widest anabolic window).(1,4–7) Furthermore, bone turnover levels usually plateau between 6 and 12 mo and subsequently decline, suggesting that the skeleton becomes resistant to ongoing TPTD administration and bone benefits might be of lesser magnitude at this latter stage of therapy. This is consistent with BMD findings, which are most marked, particularly in the spine, over the first year of therapy.(4,6,7) Therefore, it is possible that the optimal use of TPTD to enhance bone growth would be for shorter periods, with consideration of retreatment in the future, if necessary.
In patients who receive TPTD, it is necessary to follow the TPTD course with an antiresorptive agent, such as alendronate, to maintain or enhance TPTD-induced benefits.(8,9) However, there have been questions concerning the anabolic potential of TPTD in patients who have received prior alendronate that might preclude retreatment with TPTD as a viable therapeutic option. To this end, prior studies by our group and others have shown an anabolic effect of TPTD even after long-term alendronate administration,(6,10–12) but the magnitude of the BMD increment might depend on whether the previously used antiresorptive agent is continued or stopped when TPTD is started.(13) In this study, we determine whether BMD remains stable with continued alendronate over 1 yr after TPTD administration and whether retreatment with a second discrete course of TPTD (while subjects remain on alendronate) can produce another anabolic effect on the skeleton.
MATERIALS AND METHODS
Subjects
In our original protocol,(14) 126 women on prior and ongoing alendronate were randomized to continue alendronate and receive daily TPTD or cyclic TPTD (3-mo cycles) or remain on alendronate alone. One hundred eight women completed the original 15-mo treatment protocol (n = 72 women on TPTD, including 38 from the original daily group and 36 from the original cyclic group). A total of 49 women (24 from the original daily group and 25 from the original cyclic group) completed the 12 mo of follow-up on alendronate alone. At that time, women were approached to determine eligibility and interest in the retreatment study. The inclusion criteria for the retreatment protocol were completion of the original TPTD course, completion of the 12-mo follow-up on alendronate alone, and current BMD at the spine, total hip, or femoral neck ≤ −2.5 or occurrence of osteoporosis-related fractures in the prior 3 yr. A total of 32 women from one of the original TPTD groups (17 from the original daily TPTD group and 15 from the original cyclic TPTD group) agreed to participate in the extension study and receive another 15-mo course of TPTD.
Treatment protocol
All volunteers continued alendronate 70 mg once weekly and received daily TPTD 25 μg/d during the 15-mo retreatment period, in addition to a total calcium intake of 1200 mg/d through diet and supplements and vitamin D intake of at least 400–1000 IU/d, based on maintenance of serum 25(OH)D target levels above 25 ng/ml.
Both daily self-administered TPTD and oral once weekly alendronate were well tolerated in all subjects. Compliance was >80% with both medications. A total of five women withdrew during the retreatment protocol for reasons unrelated to study medication.
Assessments
Fasting serum and second void urine samples were obtained at baseline (just before TPTD retreatment) and at 3, 6, and 15 mo during TPTD retreatment. Osteocalcin (OC) was measured by immunoradiometric assay (Immutopics), and N-terminal propeptide of type I procollagen (PINP) was measured by an Osteomark ELISA (Ostex International). Baseline second-void fasting urine samples were analyzed for bone resorption by measuring the levels of urinary cross-linked N-telopeptide (NTX) with the use of an ELISA (Ostex International) and creatinine by standard automated methods. All intra-assay CVs were <8.3%, and interassay CVs were <13.7%.
BMD was measured in the spine and hip by DXA at baseline (just before TPTD retreatment) and after 15 mo of retreatment using the Lunar Prodigy (General Electric/Lunar). In vivo short-term precision was 0.7% for spine measurements and 0.9% for total hip measurements. Long-term precision (2 yr) was <1.7% for all sites.
Statistical analysis
Descriptive variables for the group entering the retreatment study were compared with the original TPTD groups and the group who did not enter the retreatment study by t-tests to determine whether the retreatment sample appeared biased.
BMD stability over the 12-mo alendronate-only interval was determined by comparing mean BMD levels in both the spine and hip at the end of the original 15-mo trial and at the end of the 12-mo alendronate-only interval by paired t-tests (for those who completed the entire 27-mo study).
Absolute change in BMD at 15 mo during TPTD retreatment was compared with absolute change at 15 mo during the first TPTD treatment using paired t-tests (for those who completed the entire study). When the original cyclic and daily groups were analyzed separately, there were no differences in results. Because the first 3-mo phase of the original protocol was identical for the daily and cyclic groups, biochemical results were pooled for the two groups for each treatment period and analyzed by signed rank tests to determine whether there were differences in the magnitude of the changes seen between the first TPTD treatment and the retreatment. Biochemical turnover indices were also compared at baseline and 15 mo during the retreatment for the original daily group, although no statistical analysis was performed comparing baseline to 15-mo biochemistry during the retreatment in the original cyclic group because the protocol was distinctly different for the original TPTD treatment versus the retreatment.
The proportion of patients above and below least significant change levels with regard to biochemical markers (21% for PINP and OC, 70% for NTX)(15) and various BMD increments (<3%, 3–6%, and >6%) were determined and compared between the first TPTD treatment and retreatment using Fisher's exact tests.
RESULTS
There were no differences in height, weight, body mass index, years on alendronate, years from menopause, or BMD comparing the group entering the retreatment study with the original TPTD groups. Furthermore, these descriptive variables did not differ between those who entered the retreatment study compared with those who did not enter the retreatment study, with the exception of BMD. BMD was significantly higher at all skeletal sites at the end of the 27-mo trial in those subjects who did not enter the retreatment protocol (consistent with the entry criteria for the retreatment study).
In the original treatment trial, mean spine BMD levels increased similarly in both daily and cyclic cohorts over 15 mo(6) and remained stable in the 49 patients who completed the subsequent 12-mo observation on alendronate alone (n = 25 from the original daily group and n = 24 from the original cyclic group). Table 1 shows descriptive characteristics, baseline biochemistry, and BMD for subjects recruited into the retreatment protocol (n = 17 from the original daily group and n = 15 from the original cyclic group). The cohorts were very similar, with a difference only for hip BMD (slightly higher at baseline in the original daily group at the time of retreatment).
Table 1.
Baseline Characteristics of the Study Population at Retreatment
| Prior daily group (n = 17) | Prior cyclic group (n = 15) | |
| Descriptive characteristics (mean ± SD) | ||
| Age (yr) | 69.1 ± 6.8 | 69.6 ± 7.4 |
| Years from menopause | 21.0 ± 10.0 | 21.2 ± 9.4 |
| Years on alendronate | 5.3 ± 1.3 | 5.2 ± 1.5 |
| Prior fracture of spine, hip, or wrist [n (%)] | 12 (71) | 8 (53) |
| BMD (mean ± SD) | ||
| Spine BMD (g/cm2) | 0.85 ± 0.09 | 0.83 ± 0.07 |
| Spine T-score | −2.9 ± 0.7 | −3.0 ± 0.6 |
| Total hip BMD (g/cm2) | 0.79 ± 0.10 | 0.72 ± 0.06 |
| Total hip T-score | −1.8 ± 0.8 | −2.4 ± 0.5 |
| Turnover markers (median, IQR) | ||
| OC (ng/ml) | 5.6, 2.7 | 4.6, 3.4 |
| P1NP (μg/liter) | 24.0, 6.0 | 20.0, 11.0 |
| NTX (nmol BCE/mMCr) | 26.0, 10.0 | 28.0, 17.0 |
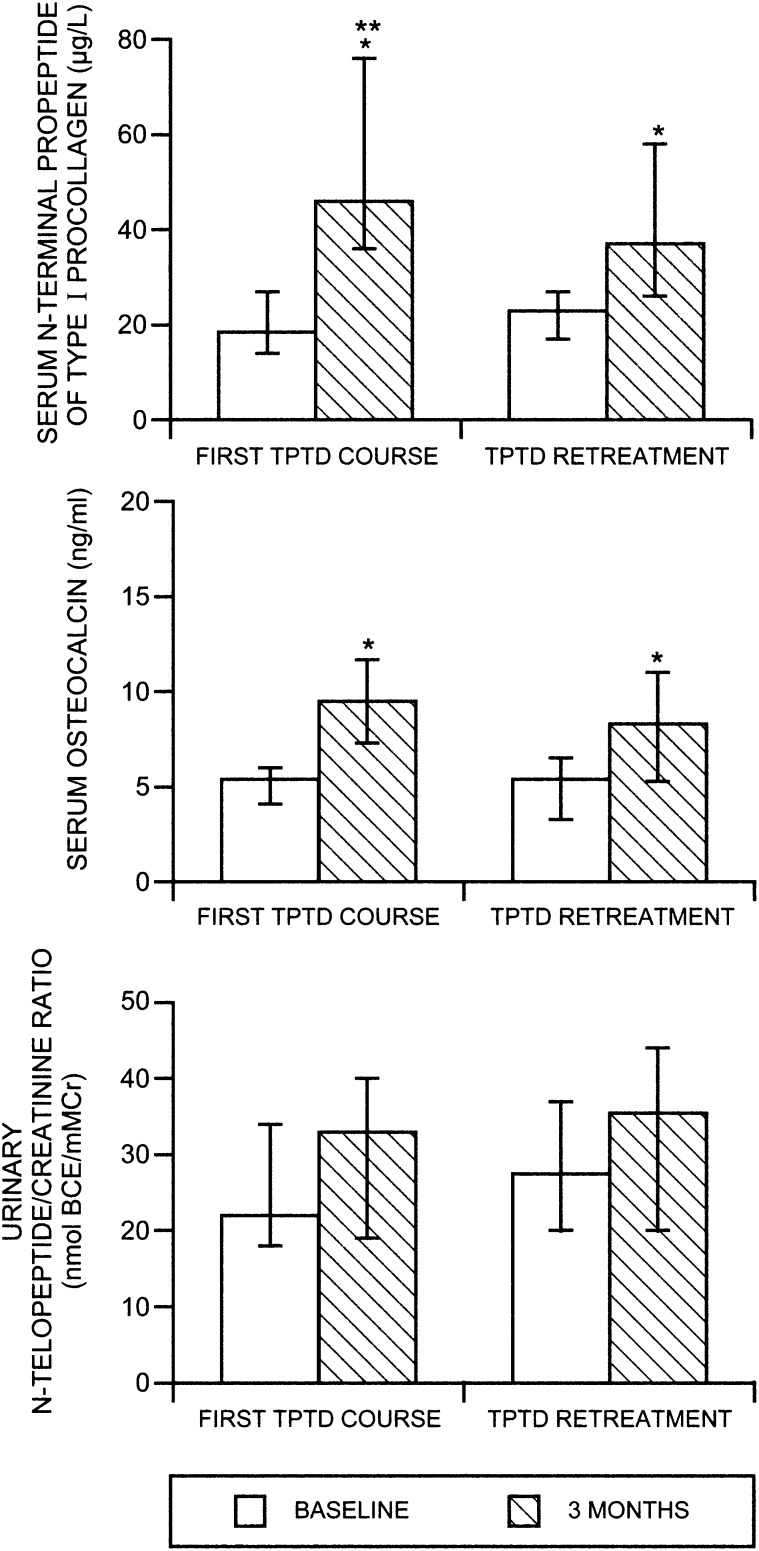
Figure 1 shows the median biochemistry values at baseline and 3 mo during the two TPTD treatment periods (original daily and cyclic groups pooled for the 3-mo analysis). Bone turnover indices increased during the first 3 mo of the TPTD retreatment similarly to what was seen during the original treatment course, although the magnitude of increments varied. Median absolute increments were 26.5 μg/liter, 4.9 ng/ml, and 3.5 nmol BCE/mM Cr above baseline (P1NP, OC, and NTX, respectively) during the original TPTD course and 11 μg/liter, 2.2 ng/ml, and 4.9 nmol BCE/mM Cr above baseline, respectively, during the retreatment. All 3-mo values were significantly different compared with their respective baselines with the exception of NTX, which did not change significantly after 3 mo in either the original TPTD course or TPTD retreatment. When comparing the original TPTD treatment versus retreatment, there were no significant differences in increments, except for P1NP, which increased significantly more with the original TPTD course compared with TPTD retreatment (p < 0.001). The proportion of subjects who had biochemical increments at 3 mo above the least significant change (LSC) for each biochemical variable during the first TPTD course (original and cyclic groups pooled) and TPTD retreatment were similar. For P1NP, 96% of subjects during the first course and 81% during the retreatment exceeded the LSC. For OC, 85% during the first course and 81% during retreatment exceeded the LSC. In contrast to the biochemical indices of bone formation, the majority of women did not exceed LSC for urinary NTX after 3 mo of TPTD administration (only 23% during the first TPTD course and 27% during TPTD retreatment). There were no significant differences in the percentages of women exceeding LSC during the first TPTD course compared with the TPTD retreatment for any of the biochemical variables.
FIG. 1.
Median levels of biochemical bone turnover indices (PINP, OC, and NTX) at baseline and 3 mo during the first TPTD course and during the TPTD retreatment (daily and cyclic groups pooled). *p < 0.001 vs. baseline; **p < 0.001 for increment during first TPTD course vs. TPTD retreatment (by signed rank test).
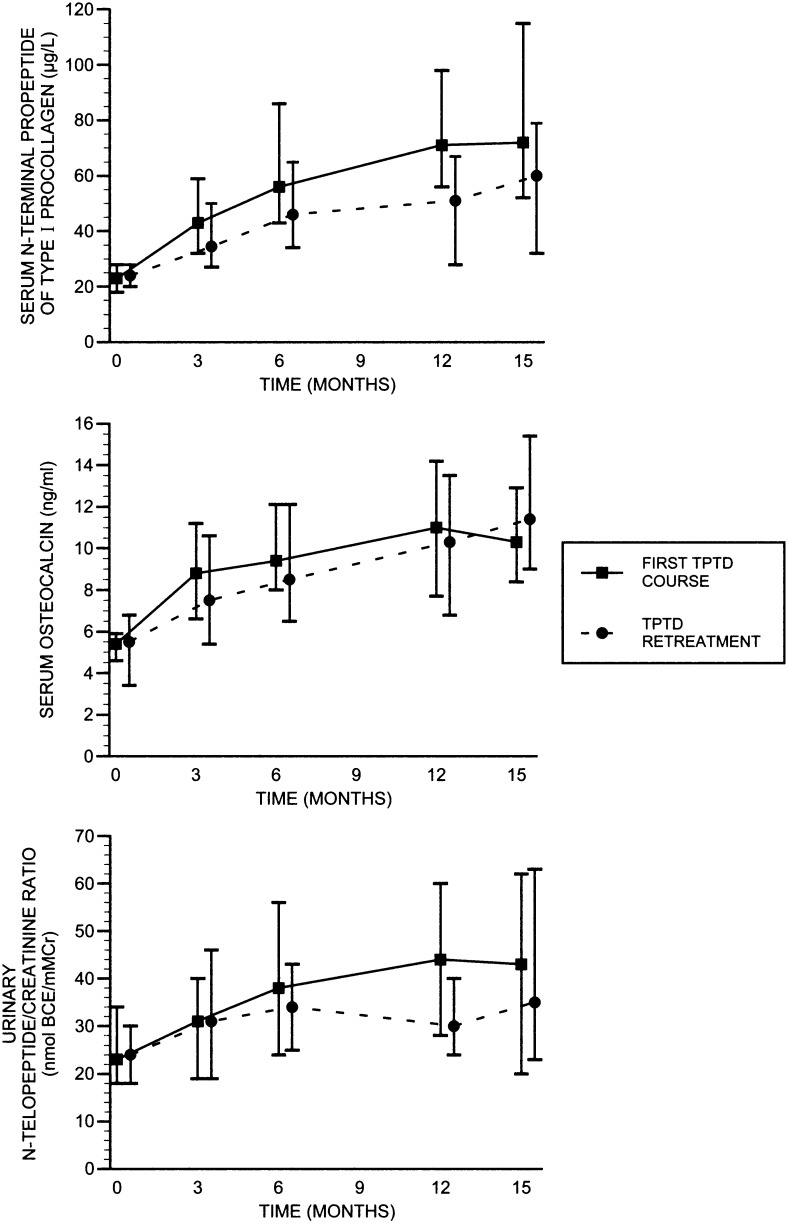
Figure 2 shows median biochemical bone turnover levels during the first 15-mo TPTD course and during the 15-mo retreatment for the original daily group. Biochemical responses were very similar during the original course and retreatment throughout the respective treatment periods. For each biochemical variable, levels reached a plateau between 9 and 15 mo, with similar levels attained during the first course and retreatment.
FIG. 2.
Median (IQR) biochemical levels during the first TPD course and during TPTD retreatment for the original daily group over 15 mo, respectively. There were no differences between the biochemical changes for OC or NTX between the first TPTD course and TPTD retreatment, but the increment for PINP was slightly lower during the TPTD retreatment vs. the first TPTD course (p = 0.04).
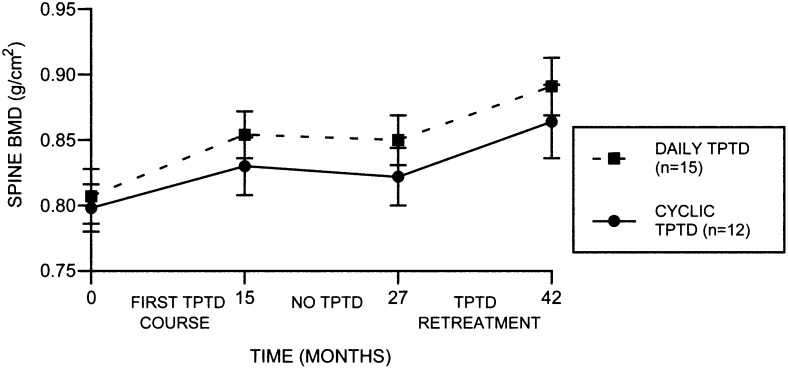
Figure 3 shows the mean spine BMD levels at baseline, at 15 mo after the original TPTD course, at 27 mo (after 12 mo on alendronate alone), and at 42 mo (after TPTD retreatment) for the original daily and original cyclic groups separately. Increments were similar during each TPTD course between the two groups and between the first TPTD course and TPTD retreatment. In the original daily group, mean spine BMD increased 0.047 ± 0.05 g/cm2 during the first TPTD course, was stable during the intervening year on alendronate alone, and increased 0.040 ± 0.03 g/cm2 during TPTD retreatment. In the original cyclic group, average spine BMD increased 0.033 ± 0.04 g/cm2 during the first TPTD course and 0.042 ± 0.04 g/cm2 during TPTD retreatment. There were no significant differences in hip BMD after TPTD retreatment in either the daily or the cyclic group (data not shown).
FIG. 3.
Mean spine BMD throughout the original TPTD course and the TPTD retreatment for the original daily (n = 15) and cyclic (n = 12) groups for all subjects who completed the full 42-mo protocol. Increments were significantly above baseline for the spine during the first TPTD course and retreatment in both groups (p ≤ 0.02 for both). There were no significant differences between increments seen during the first TPTD course and retreatment TPTD course in either of the groups.
The majority of women had spine BMD increases >3% (63% during the first TPTD course and 74% during TPTD retreatment). An increase in spine BMD that exceeded 6% was seen in 41% of subjects during the first course and 33% during retreatment.
TPTD retreatment was well tolerated. There were no withdrawals because of treatment emergent adverse events. Only one subject had minimally elevated serum calcium on one determination with return to normal spontaneously, without intervention, on the next determination.
DISCUSSION
Our results indicate that, in patients on established alendronate, who have had an initial treatment with TPTD, retreatment with TPTD (after a 12-mo interval on alendronate alone) stimulates an increase in bone formation and produces an average spine BMD increase similar to those seen during the first TPTD administration. Our findings suggest that patients who remain at high risk of fracture, despite a first course of TPTD, might benefit from a second discrete TPTD retreatment course given 12 mo later, with ongoing alendronate during and in between the two TPTD treatments.
The optimal length of treatment for TPTD remains unknown but may very well be <18–24 mo. TPTD effects on bone biochemistry are most marked during early administration (within 12 mo), and histomorphometric data confirm that the dramatic stimulation of bone formation seen early on with TPTD(16,17) is no longer present at 18 mo.(18,19) To avoid this apparent skeletal tachyphylaxis to TPTD, we suggested administering TPTD in repeated cycles of shorter duration. Because BMD is lost quickly in the absence of antiresorptive therapy after TPTD,(8,9) bisphosphonates must be administered after TPTD to maintain BMD benefits. Therefore, the concept of TPTD retreatment depends on the anabolic potential of TPTD in the setting of established bisphosphonate treatment. Preliminary data from histomorphometric studies do suggest that when TPTD is given to women on prior long-term and continuing alendronate, structural benefits are seen(20) and bone formation is stimulated dramatically.(21) We have now shown biochemical and BMD benefits with short 3-mo cycles of TPTD(6) and longer 15-mo cycles of TPTD in women on prior and ongoing alendronate in this paper.
There is no way to determine from this trial whether continued cyclic administration of TPTD throughout the 42 mo may have been better than the approach used here: cyclic followed by daily administration. The continued utilization of cyclic therapy for 4 yr (comprising a total cumulative therapy duration of 2 yr) is being pursued in a separate ongoing study at our center.
Why the skeleton becomes resistant to the effects of ongoing TPTD administration is unknown. Downregulation of PTH receptors has not been shown but is a possible explanation; however, the time course for resistance to TPTD seems too delayed for this to be a likely explanation. Resistance could possibly be related to a TPTD-induced increase in osteocyte number or density(22) with increased production of sclerostin subsequently reducing further osteoblast formation.(23,24) Alternatively, or additionally, it may be that the precursor pool for osteoblasts is exhausted after ∼12–18 mo. Waiting for an additional 12 mo to allow the regeneration of the osteoblast precursor pool, while holding BMD stable on alendronate, is a viable approach to obviate this problem. Furthermore, if sclerostin-induced inhibition of osteoblast formation is involved in resistance to ongoing TPTD administration, reduced new bone formation during the intervening period off TPTD might be associated with lower osteocyte production and a renewed ability to respond to retreatment with TPTD. It is completely unknown, however, whether 12 mo off TPTD would be the optimal duration in between the discrete TPTD treatments.
In conclusion, TPTD is the only currently available anabolic compound that has the potential to produce greater improvements in bone strength than a potent antiresorptive medication(25–27); however, there are many questions that remain regarding its optimal use. Whereas our study is small and observational, it provides support for the concept that women who have had long-term and continuing bisphosphonate treatment can still manifest an anabolic response to TPTD (assessed by biochemistry and BMD). Women who remain at high risk of fracture after receiving a first course of TPTD treatment and continuing their bisphosphonate could be considered for a second discrete retreatment with TPTD ∼1 yr later. Our results indicate that retreatment with TPTD (after a 12-mo interval on alendronate alone) will stimulate an increase in bone formation and produce an average spine BMD increase similar to those seen during the first TPTD administration.
Footnotes
Dr. Cosman serves as a consultant for Eli Lilly, Merck, and Novartis. Dr. Lindsay has served as a consultant for Procter & Gamble, NPS, Sanofi-Aventis, Roche-GlaxoSmithKline, Novartis, and Wyeth. All other authors state that they have no conflicts of interest.
REFERENCES
- 1.Eli Lilly. IN, USA: Eli Lilly and Company, Indianapolis; 2002. Forteo Package Insert. [Google Scholar]
- 2.Vahle JL, Long GG, Sandusky G, Westmore M, Ma YL, Sato M. Bone neoplasms in F344 rats given teriparatide [rhPTH(1-34)] are dependent on duration of treatment and dose. Toxicol Pathol. 2004;32:426–438. doi: 10.1080/01926230490462138. [DOI] [PubMed] [Google Scholar]
- 3.Vahle JL, Sato M, Long GG, Young JK, Francis PC, Engelhardt JA, Westmore MS, Linda Y, Nold JB. Skeletal changes in rats given daily subcutaneous injections of recombinant human parathyroid hormone (1-34) for 2 years and relevance to human safety. Toxicol Pathol. 2002;30:312–321. doi: 10.1080/01926230252929882. [DOI] [PubMed] [Google Scholar]
- 4.Lindsay R, Nieves J, Formica C, Henneman E, Woelfert L, Shen V, Dempster D, Cosman F. Randomised controlled study of effect of parathyroid hormone on vertebral-bone mass and fracture incidence among postmenopausal women on oestrogen with osteoporosis. Lancet. 1997;350:550–555. doi: 10.1016/S0140-6736(97)02342-8. [DOI] [PubMed] [Google Scholar]
- 5.McClung MR, San Martin J, Miller PD, Civitelli R, Bandeira F, Omizo M, Donley DW, Dalsky GP, Eriksen EF. Opposite bone remodeling effects of teriparatide and alendronate in increasing bone mass. Arch Intern Med. 2005;165:1762–1768. doi: 10.1001/archinte.165.15.1762. [DOI] [PubMed] [Google Scholar]
- 6.Cosman F, Nieves J, Zion M, Woelfert L, Luckey M, Lindsay R. Daily and cyclic parathyroid hormone in women receiving alendronate. N Engl J Med. 2005;353:566–575. doi: 10.1056/NEJMoa050157. [DOI] [PubMed] [Google Scholar]
- 7.Cosman F, Nieves J, Woelfert L, Formica C, Gordon S, Shen V, Lindsay R. Parathyroid hormone added to established hormone therapy: Effects on vertebral fracture and maintenance of bone mass after parathyroid hormone withdrawal. J Bone Miner Res. 2001;16:925–931. doi: 10.1359/jbmr.2001.16.5.925. [DOI] [PubMed] [Google Scholar]
- 8.Black DM, Bilezikian JP, Ensrud KE, Greenspan SL, Palermo L, Hue T, Lang TF, McGowan JA, Rosen CJ. One year of alendronate after one year of parathyroid hormone (1-84) for osteoporosis. N Engl J Med. 2005;353:555–565. doi: 10.1056/NEJMoa050336. [DOI] [PubMed] [Google Scholar]
- 9.Lindsay R, Scheele WH, Neer R, Pohl G, Adami S, Mautalen C, Reginster JY, Stepan JJ, Myers SL, Mitlak BH. Sustained vertebral fracture risk reduction after withdrawal of teriparatide in postmenopausal women with osteoporosis. Arch Intern Med. 2004;164:2024–2030. doi: 10.1001/archinte.164.18.2024. [DOI] [PubMed] [Google Scholar]
- 10.Boonen S, Marin F, Obermayer-Pietsch B, Simoes ME, Barker C, Glass EV, Hadji P, Lyritis G, Oertel H, Nickelsen T, McCloskey EV. Effects of previous antiresorptive therapy on the bone mineral density response to two years of teriparatide treatment in postmenopausal women with osteoporosis. J Clin Endocrinol Metab. 2008;93:852–860. doi: 10.1210/jc.2007-0711. [DOI] [PubMed] [Google Scholar]
- 11.Ettinger B, San Martin J, Crans G, Pavo I. Differential effects of teriparatide on BMD after treatment with raloxifene or alendronate. J Bone Miner Res. 2004;19:745–751. doi: 10.1359/JBMR.040117. [DOI] [PubMed] [Google Scholar]
- 12.Miller PD, Delmas PD, Lindsay R, Watts NB, Luckey M, Adachi J, Saag K, Greenspan SL, Seeman E, Boonen S, Meeves S, Lang TF, Bilezikian JP. Early responsiveness of women with osteoporosis to teriparatide following therapy with alendronate or risedronate. J Clin Endocrinol Metab. 2008;93:3785–3793. doi: 10.1210/jc.2008-0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cosman F, Wermers RA, Recknor C, Mauck KF, Xie L, Glass EV, Krege JH. Efficacy of adding teriparatide versus switching to teriparatide in postmenopausal women with osteoporosis previously treated with raloxifene or alendronate. J Bone Miner Res. 2007;22:S1–S127. [Google Scholar]
- 14.Dempster DW, Hughes-Begos CE, Plavetic-Chee K, Brandao-Burch A, Cosman F, Nieves J, Neubort S, Lu SS, Iida-Klein A, Arnett T, Lindsay R. Normal human osteoclasts formed from peripheral blood monocytes express PTH type 1 receptors and are stimulated by PTH in the absence of osteoblasts. J Cell Biochem. 2005;95:139–148. doi: 10.1002/jcb.20388. [DOI] [PubMed] [Google Scholar]
- 15.Hannon R, Blumsohn A, Naylor K, Eastell R. Response of biochemical markers of bone turnover to hormone replacement therapy: Impact of biological variability. J Bone Miner Res. 1998;13:1124–1133. doi: 10.1359/jbmr.1998.13.7.1124. [DOI] [PubMed] [Google Scholar]
- 16.Lindsay R, Cosman F, Zhou H, Bostrom MP, Shen VW, Cruz JD, Nieves JW, Dempster DW. A novel tetracycline labeling schedule for longitudinal evaluation of the short-term effects of anabolic therapy with a single iliac crest bone biopsy: Early actions of teriparatide. J Bone Miner Res. 2006;21:366–373. doi: 10.1359/JBMR.051109. [DOI] [PubMed] [Google Scholar]
- 17.Lindsay R, Zhou H, Cosman F, Nieves J, Dempster DW, Hodsman AB. Effects of a one-month treatment with parathyroid hormone (1-34) on bone formation on cancellous, endocortical and periosteal surfaces of the human ilium. J Bone Miner Res. 2007;4:495–502. doi: 10.1359/jbmr.070104. [DOI] [PubMed] [Google Scholar]
- 18.Dempster DW, Cosman F, Kurland ES, Zhou H, Nieves J, Woelfert L, Shane E, Plavetic K, Muller R, Bilezikian J, Lindsay R. Effects of daily treatment with parathyroid hormone on bone microarchitecture and turnover in patients with osteoporosis: A paired biopsy study. J Bone Miner Res. 2001;16:1846–1853. doi: 10.1359/jbmr.2001.16.10.1846. [DOI] [PubMed] [Google Scholar]
- 19.Jiang Y, Zhao JJ, Mitlak BH, Wang O, Genant HK, Eriksen EF. Recombinant human parathyroid hormone (1-34) [teriparatide] improves both cortical and cancellous bone structure. J Bone Miner Res. 2003;18:1932–1941. doi: 10.1359/jbmr.2003.18.11.1932. [DOI] [PubMed] [Google Scholar]
- 20.Li J, Burr DB, Stepan JJ, Fahrleitner-Pammer A, Sipos A, Mullarney T, Westmore M, Dobnig H, Sato M, Pavo I. Teriparatide improves bone michoarchitecture in postmenopausal women previously treated with alendronate. J Bone Miner Res. 2007;22:S1–S28. [Google Scholar]
- 21.Lindsay R, Cosman F, Zhou H, Nieves J, Bostrom MP, Barbuto N, Dempster D. Prior alendronate treatment does not inhibit the early estimulation of osteoblast activity in response to teriparatide. J Bone Miner Res. 2007;22:S1–S124. [Google Scholar]
- 22.Manolagas SC, Weinstein RS, Jilka RL, Parfitt AM. Parathyroid hormone and corticosteroid-induced osteoporosis. Lancet. 1998;352:1940. doi: 10.1016/S0140-6736(05)60443-6. [DOI] [PubMed] [Google Scholar]
- 23.Baron R, Rawadi G. Wnt signaling and the regulation of bone mass. Curr Osteoporos Rep. 2007;5:73–80. doi: 10.1007/s11914-007-0006-0. [DOI] [PubMed] [Google Scholar]
- 24.Baron R, Rawadi G. Targeting the Wnt/beta-catenin pathway to regulate bone formation in the adult skeleton. Endocrinology. 2007;148:2635–2643. doi: 10.1210/en.2007-0270. [DOI] [PubMed] [Google Scholar]
- 25.Keaveny TM, Donley DW, Hoffmann PF, Mitlak BH, Glass EV, San Martin JA. Effects of teriparatide and alendronate on vertebral strength as assessed by finite element modeling of QCT scans in women with osteoporosis. J Bone Miner Res. 2007;22:149–157. doi: 10.1359/jbmr.061011. [DOI] [PubMed] [Google Scholar]
- 26.Keaveny TM, Hoffmann PF, Kopperdahl DL, Donley DW, Krohn K, Glass EV, Mitlak BH. Comparison of the effects of teriparatide and alendronate on parameters of total hip strength as assessed by finite element analysis: Results from the Forteo and Alendronate Comparison Trial. J Bone Miner Res. 2007;22:S1–S26. doi: 10.1359/jbmr.061011. [DOI] [PubMed] [Google Scholar]
- 27.Keaveny TM, Hoffmann PF, Singh M, Palermo L, Bilezikian JP, Greenspan SL, Black DM. Femoral bone strength and its relation to cortical and trabecular changes after treatment with PTH, alendronate, and their combination as assessed by finite element analysis of quantitative CT scans. J Bone Miner Res. 2008;23:1974–1982. doi: 10.1359/JBMR.080805. [DOI] [PMC free article] [PubMed] [Google Scholar]