Abstract
The goal of this study was to examine the expression and regulation of aquaporin2 (AQP2), a tonicity-sensitive water channel in nucleus pulposus cells of the intervertebral disc. We found that AQP2 protein was expressed in vivo in both rat and human discs. We determined whether AQP2 promoter expression was regulated by osmolarity in a tonicity enhancer binding protein (TonEBP)-dependent manner. When TonEBP was suppressed under hypertonic conditions or overexpressed under isotonic conditions, AQP2 promoter activity was correspondingly inhibited or induced. The role of TonEBP in controlling AQP2 expression was confirmed using mouse embryonic fibroblasts (MEFs) derived from TonEBP-null mice. We studied whether calcium in addition to osmolarity played a role in regulation of AQP2 in nucleus pulposus cells. We also determined whether both TonEBP and calcineurin–nuclear factor of activated T cells (NFAT) signaling contributed to ionomycin, a calcium ionophore, mediated induction of AQP2. Co-transfection of AQP2 reporter with calcineurin (CnA/B) and/or NFAT1–4 vectors suggested that this pathway did not control AQP2 promoter activity in nucleus pulposus cells. These findings were also validated using MEFs from TonEBP, fibroblasts from CnAα- and CnAβ-null mice, and mutant TonE reporter constructs. Results of these studies suggest that, in nucleus pulposus cells, osmotic pressure and calcium modulate AQP2 expression through TonEBP and are independent of the calcineurin–NFAT pathway. Because calcium flux reflects a change in applied stress, the possibility exists that NFAT5/TonEBP modulate not just water balance in the disc but also accommodate applied biomechanical forces.
Key words: intervertebral disc, nucleus pulposus, aquaporin2, tonicity enhancer binding protein, osmolarity, calcium, nuclear factor of activated T cells
INTRODUCTION
The intervertebral disc is a specialized biomechanical structure that permits movement between adjacent vertebrae and accommodates applied compressive forces. It consists of an outer ligamentous annulus fibrosus that encloses a gel-like tissue, the nucleus pulposus. Whereas sparse, cells in the nucleus pulposus secrete a complex extracellular matrix that primarily contains collagen type II and the proteoglycan aggrecan. The numerous charged glycosaminoglycan side-chains of the aggrecan molecule interact with cations, thereby raising the osmolarity and the water content of the disc tissues.(1–4) The unique hydration properties of the nucleus pulposus promotes dynamic loading and unloading, permitting the spine to contain large shifts in biomechanical forces. However, the mechanisms by which these cells accommodate changes in osmolarity and fluid flow have received little attention.
One of the primary responses of disc cells to variations in local osmolarity is a change in regulatory cell volume. Disc cells and chondrocytes alike adapt to these osmotic shifts by remodeling their cytoskeleton and by catalyzing the transport of osmotically active molecules and water across the plasma membrane.(5–8) Water transport across the cell membrane is regulated by a large family of channel-forming proteins: the aquaporins (AQPs).(9) AQP2, an arginine vasopressin regulated channel, plays an important role in water reabsorption by connecting tubules and collecting ducts of the kidney.(10) When activated, phosphorylation of critical serine residues in AQP2 results in its translocation from cytoplasmic vesicles to the apical membrane. Intercalated with membrane proteins, AQP2 enhances water influx into the cell.(10) Recent studies have suggested that, in the kidney, expression of AQP2 is regulated by tonicity enhancer binding protein (TonEBP), also called nuclear factor of activated T cells (NFAT)5.(11–13) Studies by Li et al.(12) suggested that calcium ions with calcineurin–NFAT participate in regulation of AQP2 expression. Related to the functional importance of this system, Pritchard et al.(5) have documented the presence of calcium transients in disc cells exposed to osmotic stress. Whether the NFAT5 calcineurin signaling system serves to adapt nucleus pulposus cells to these transients has not been delineated.
The objective of this study was to determine the role of AQP2 in regulating the hydration status of cells of the intervertebral disc. Specifically, we asked the following question: do the nucleus pulposus cells express AQP2 and is their expression regulated by disc osmolarity and calcium? Results of this study clearly show that, in both the rat and the human, nucleus pulposus cells express AQP2 protein. Importantly, our data indicates that osmotic pressure and calcium modulate AQP2 expression through TonEBP and is independent of the calcineurin–NFAT signaling pathway. This finding lends credence to the view that, by regulating the hydration status of the disc, TonEBP maintains cell function in a hyperosmotic mechanically stressed environment.
MATERIALS AND METHODS
Reagents and plasmids
Rabbit polyclonal TonEBP antibody was a kind gift from Dr. H. Moo Kwon, University of Maryland. Wildtype (WT) AQP2, and mutant (MT) AQP2 luciferase reporters were provided by Dr. Feng Chen, Washington University, St. Louis, MO, USA.(12) Plasmids were kindly provided by Dr. Takashi Ito, Osaka University, Osaka, Japan (taurine transporter [TauT] [WT], TauT [MT] reporter),(14) Dr. Ben C. Ko, University of Hong Kong, China (FLAG-DN-TonEBP, FLAG-TonEBP, and FLAG-CMV2),(15) Dr. Gerald Crabtree, Stanford University (CnA and CnB), Dr. Jeffery Molkentin, Cincinnati Children's Hospital Medical Center (NFAT4 and pECE), and Dr. Tania Crotti, Harvard Institutes of Medicine (NFAT2). Plasmids for NFAT1 (11100) and CA-NFAT2 (11102), developed by Dr. Anjana Rao,(16) NFAT3 (10961), developed by Dr. Toren Finkel,(17) and 3xNFAT-Luc (17870), developed by Dr. Gerald Crabtree,(18) were obtained from Addgene (Cambridge, MA, USA). As an internal transfection control, vector pRL-TK (Promega) containing Renilla reniformis luciferase gene was used. The amount of transfected plasmid, the pretransfection period after seeding, and the post-transfection period before harvesting were optimized for rat nucleus pulposus cells using pSV β-galactosidase plasmid (Promega).(19)
Human tissue specimens
Human tissues were collected as surgical waste during spinal surgical procedures. In line with Thomas Jefferson University's Institutional Review Board guidelines, informed consent for sample collection was obtained for each patient. Assessment of the disease state was performed using the modified Thompson grading.(20)
Immunohistological studies
Freshly isolated rat spines or human discal tissues were immediately fixed in 4% wt/vol paraformaldehyde in PBS and embedded in paraffin. Transverse and coronal sections, 6–8 μm in thickness, were deparaffinized in xylene and rehydrated through graded ethanol. A few sections were stained with alcian blue, eosin, and hematoxylin. For localizing AQP2, sections were incubated with the anti-AQP2 antibody (Alamone Laboratories, Haifa, Israel, or Calbiochem) in 2% wt/vol BSA in PBS at a dilution of 1:200 at 4°C overnight. After thoroughly washing the sections, the bound primary antibody was incubated with biotinylated universal secondary antibody at a dilution of 1:20 (Vector Laboratories) for 10 min at room temperature or biotinylated goat anti-rabbit (1:200) for 45 min. Sections were incubated with a streptavidin/peroxidase complex for 5 min and washed with PBS, and color was developed using 3′-3-diaminobenzidine (Vecta Stain Universal Quick Kit; Vector Laboratories). AQP2-labeled human tissue sections were counterstained with hematoxylin. Preimmunne rabbit IgG (1:200) was used as an isotype control.
Isolation of rat nucleus pulposus cells
Nucleus pulposus cells were isolated from the rat spine using the method reported earlier(19,21) and approved by the Institutional Animal Care Committee of Thomas Jefferson University. Briefly, male Wistar rats, (250 g) were killed with CO2, and lumbar intervertebral discs were removed from the spinal column. The gel-like nucleus pulposus was isolated, using a dissecting microscope, and treated with 0.1% wt/vol collagenase and 10 U/ml hyaluronidase for 4–6 h. This procedure partially digested the tissue and thereby enhanced the subsequent release of cells trapped in the dense matrix. The partially digested tissue was maintained as an explant in DMEM and 10% FBS supplemented with antibiotics. Nucleus pulposus cells migrated out of the explant after 1 wk. When confluent, the cells were lifted using a trypsin (0.25%) EDTA (1 mM) solution and subcultured in 10-cm dishes.
Knockout cells
TonEBP/NFAT5 wildtype and null mouse embryonic fibroblasts (MEFs; originally from Dr. Steffan N. Ho), were provided by Dr. Feng Chen, Washington University, St. Louis, MO, USA.(12) Primary medullary fibroblasts derived from CnAα-null, CnAβ-null, and CnA wildtype mice were provided by Dr. Jennifer Gooch, Emory University. These cells were used as control cells for some experiments.
Immunofluorescence microscopy
Cells were plated in flat-bottomed 96-well plates (5000 cells/well). After 24-h culture, cells were fixed with 4% vol/vol paraformaldehyde, permeabilized with 0.2% Triton-X 100 in PBS for 10 min, blocked with PBS containing 5% vol/vol FBS, and incubated with anti-AQP2 (1:200; BD Biosciences) antibody at 4°C overnight. As a negative control, cells were reacted with isotype IgG under similar conditions. After washing, the cells were incubated with Alexa fluor-488–conjugated anti-mouse secondary antibody (Molecular Probes, St. Louis, MO, USA), at a dilution of 1:50 for 1 h at room temperature. Cells were washed and imaged using a laser scanning confocal microscope (Fluoview; Olympus).
Real-time RT-PCR analysis
At the end of treatment, total RNA was extracted from nucleus pulposus cells using RNAeasy mini columns (Qiagen). Before elution from the column, RNA was treated with RNase free DNase I. One hundred nanograms total RNA was used as template for real-time PCR analysis. Reactions were set up in microcapillary tubes using 1 μl RNA with 9 μl of a LightCycler FastStart DNA Master SYBR Green I mix (Roche Diagnostics, Indianapolis, IN, USA), to which gene-specific forward and reverse PCR primers were added (AQP2: NCBI NM_012909; forward: 5′-tgtcaatgctctccacaacaacgc-3′: 435–459 bp, reverse: 5′-aaacttgccagtgacaactgctgg-3′: 655–678 bp). Each set of samples included a template-free control. PCR reactions were performed in a LightCycler (Roche) according to the manufacturer's instructions. All the primers used were synthesized by Integrated DNA Technologies(Coralville, IA, USA).
Western blotting
Total cell lysates were resolved on 8% wt/vol or 10% wt/vol SDS-polyacrylamide gels. Proteins were transferred by electroblotting to PVDF membranes (Bio-Rad). The membranes were blocked with 5% wt/vol nonfat dry milk in TBST (50 mM Tris, pH 7.6, 150 mM NaCl, 0.1% vol/vol Tween 20) and incubated overnight at 4°C in 3% wt/vol nonfat dry milk in TBST with antibodies against AQP2 (Alamone Laboratories or BD Biosciences, 1:1000), β-tubulin (1:1000; DSHB, Iowa City, IA). Immunolabeling was detected using the ECL reagent (Amersham Biosciences).
Transfections and dual luciferase assay
Nucleus pulposus cells or WT and null MEFs were transferred to 24-well plates at a density of 5.0–6.0 × 104 cells/well 1 day before transfection. LipofectAMINE 2000 (Invitrogen) or Transgater (America Pharma Source, Gaithersburg, MD, USA) was used as a transfection reagent. For each transfection, desired concentrations and combination of plasmids were premixed with the transfection reagent. In some experiments, 24 h after transfection, cells were cultured in hypertonic media (410–500 mosmol/kg) or treated with ionomycin (1 μM) and phorbol myrystate acetate (PMA) (100 ng) with or without FK506 (10 ng/ml) and cyclosporine A (1 μg/ml) or BAPTA-AM (10 μM). The next day, the cells were harvested, and a Dual-Luciferase reporter assay system (Promega) was used for sequential measurements of firefly and Renilla luciferase activities. Quantification of luciferase activities and calculation of relative ratios were carried out using a luminometer (TD-20/20; Turner Designs, Sunnyvale, CA, USA). At least three independent transfections were performed, and all analyses were carried out in triplicate.
Statistical analysis
All measurements were performed in triplicate; data are presented as mean ± SD. Differences between groups were analyzed by Student's t-test (p< 0.05).
RESULTS
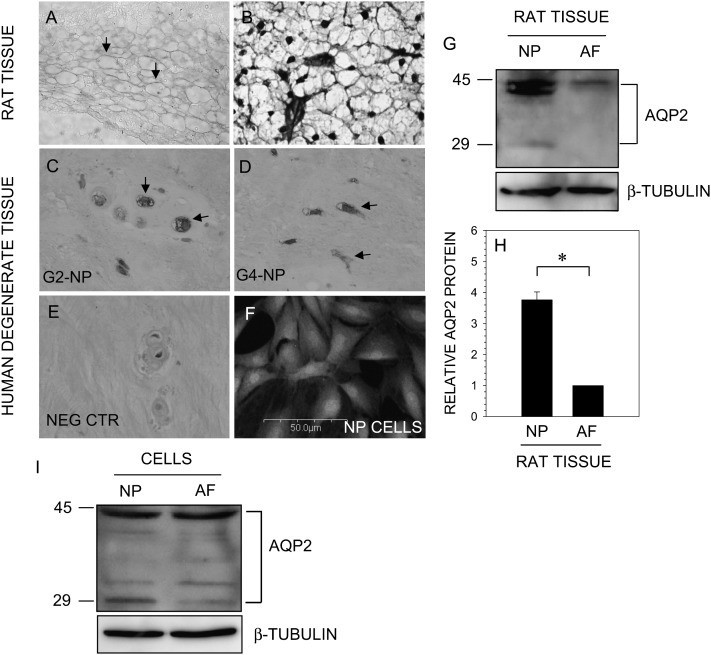
Sagittal sections of the neonatal rat (Fig. 1A) and degenerate human discs (Figs. 1C and 1D) were stained with an antibody to AQP2 or isotype control (Fig. 1E). Some sections were also counterstained with H&E and alcian blue for morphology assessment (Fig. 1B). AQP2 is expressed by cells of the nucleus pulposus in rat and human discs (Figs. 1A, 1C, and 1D). In both cases, staining is localized to the plasma membrane. In human tissue, some cells showed an intense labeling, whereas others showed weaker AQP2 expression. Moreover, staining is also seen in the cytosol of human nucleus pulposus cells (Figs. 1C and 1D). Whereas some variability in staining is observed in the human tissue sections, this probably is caused by the degree of degeneration of the disc, inherent biological variations between individuals, and heterogeneity in cell populations within the tissue. There is weak expression of AQP2 protein in the inner annulus fibrosus cells of the rat disc (data not shown). In addition, we studied expression of AQP2 in cultured rat nucleus pulposus cells using immunofluorescence microscopy. Similar to the native tissues, nucleus pulposus in culture express AQP2 protein (Fig. 1F).
FIG. 1.
AQP2 is expressed by the disc tissue. Sections of the intervertebral disc of neonatal rat (A and B) and human (C–E) stained with an antibody against AQP2. Sections were treated with anti-AQP2 antibody (A, C, and D), stained with H&E and alcian blue (B), or treated with isotype antibody (E). Note that nucleus pulposus (NP) cells express AQP2 protein (A, C, and D; arrows) Magnification, ×20 and ×40. (F) Immunofluorescent detection of AQP2 in cultured NP cells. Cells were treated with an antibody to AQP2, and cell nuclei were stained with propidium iodide (PI). NP cells express AQP2. Magnification, ×20. (G) Western blot analysis of AQP2 expression by NP and annulus fibrosus (AF) tissue. Note, strong expression of the 42-kDa AQP2 band in tissue extracts. (H) Multiple blots were quantified by densitometric analysis. The native NP tissue expressed higher AQP2 protein levels than AF tissue. (I) AQP2 expression by cultured NP and AF cells. Cellular proteins were separated by SDS-PAGE and analyzed by Western blot using an antibody to AQP2 and β-tubulin. Note the prominent expression of AQP2 by NP and AF cells.
We probed expression levels of AQP2 in native rat disc tissues using Western blot analysis. Figure 1G indicates that nucleus pulposus tissue expresses a prominent 40- to 42-kDa band and a weaker 29-kDa AQP2 band, whereas only the 42-kDa AQP2 band was detectable in rat annulus fibrosus tissue. Moreover, the expression level of AQP2 in nucleus pulposus tissue is significantly higher than the annulus fibrosus as seen from densitometric analysis (Fig. 1H). Similar to native tissue, cultured rat disc cells express AQP2 protein and exhibit a similar pattern of expression (Fig. 1I).
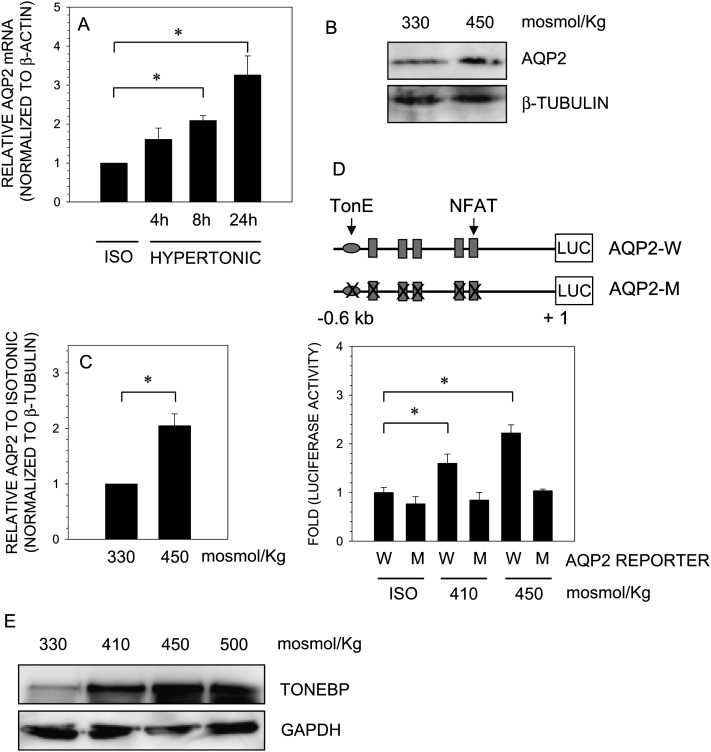
We next examined the effect of osmolarity on expression of AQP2 in nucleus pulposus cells. When cells are cultured under hypertonic conditions (450 mosmol/kg), there is a time-dependent increase in AQP2 mRNA expression (Fig. 2A). Compared with isoosmotic expression, AQP2 mRNA increase is significantly different as early as 8 h and is further increased at 24 h. Hypertonicity resulted in a concomitant increase in AQP2 protein levels (Figs. 2B and 2C). To study the effect of tonicity on the regulation of AQP2 in nucleus pulposus cells, we used a 0.6-kb rat AQP2 luciferase wildtype reporter construct and a construct containing mutations in TonEBP and NFAT binding sites (Fig. 2D). Culture of nucleus pulposus cells in hypertonic media results in an osmolarity-dependent increase in activity of the wildtype AQP2 reporter (Fig. 2D). Predictably, hypertonicity failed to induce a rise in the activity of the mutant AQP2 construct. In a parallel study, we measured the effect of hyperosmolarity on TonEBP protein expression in nucleus pulposus cells. As expected, a robust osmolarity-dependent increase in TonEBP protein expression is evident (Fig. 2E).
FIG. 2.
Effect of osmolarity on AQP2 expression in nucleus pulposus cells. Cells were cultured in hypertonic medium (450 mosmol/kg) for 4–24 h, and AQP2 expression was analyzed. (A) Real-time RT-PCR analysis of AQP2 expression indicated a time-dependent increase in mRNA expression under hypertonic conditions. (B) Western blots analysis showed there was osmotic induction in AQP2 protein expression in NP cells. (C) Multiple Western blots of AQP2 quantified by densitometric analysis. Note NP cells exhibit induction in AQP2 levels under hypertonic conditions (D) Cartoon showing AQP2 promoter constructs used for transfections. Mutant construct (AQP2-M) contains point mutations in TonEBP and all NFAT binding motif, whereas wildtype (AQP2-W) construct contains native site. NP cells were transfected with wildtype and mutant AQP2 reporter constructs along with pRL-TK vector. Cells were cultured under isotonic or increasingly hypertonic (410–450 mosmol/kg) conditions for 24 h, and luciferase reporter activity was measured. NP cells showed an osmolarity-dependent increase in wildtype promoter activity. Mutant vector did not change its activity under hypertonic conditions. (E) Western blot analysis of the expression of TonEBP protein by NP cells at 330–500 mosmol/kg. An increase in medium osmolarity from isotonic to 500 mosmol/kg resulted in a robust increase in TonEBP protein. Quantitative data represents mean ± SD of three independent experiments (n = 3); *p < 0.05; ns = nonsignificant.
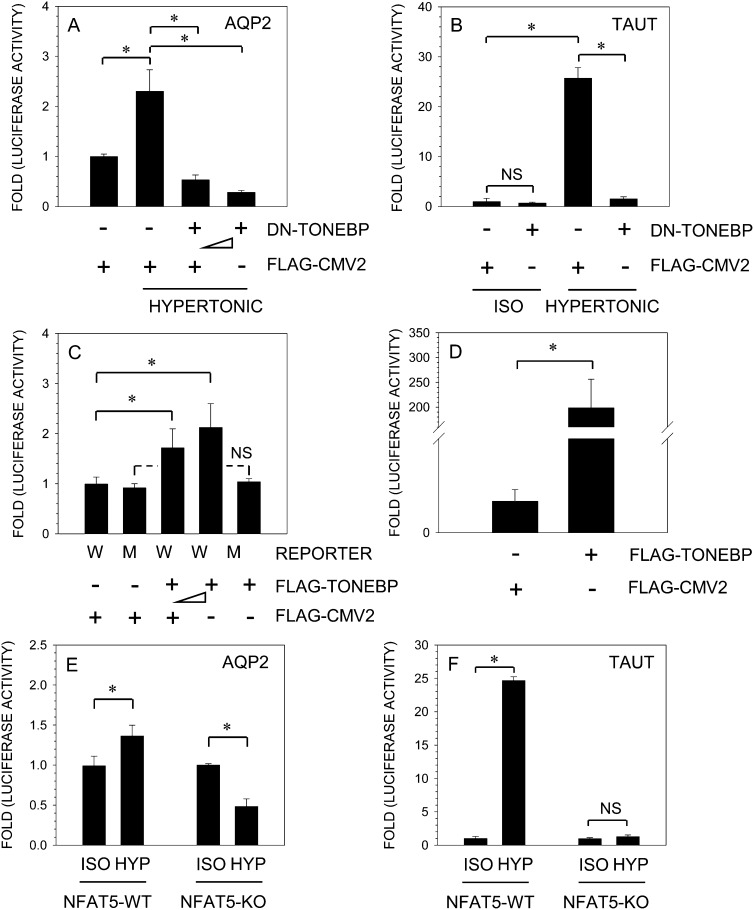
To study whether TonEBP is needed for the induction of AQP2 promoter activity, nucleus pulposus cells were transiently co-transfected with plasmids encoding DN-TonEBP and full-length TonEBP. Figure 3 shows that forced expression of DN-TonEBP completely abolishes hypertonic induction of AQP2 (A) and TauT (B) promoter activity. When the medium is hypertonic, the level of AQP2 activity in cells transfected with DN-TonEBP is lower than expression under isotonic condition. A significant inhibitory effect of DN-TonEBP expression on AQP2 reporter activity is seen at a dose of 100 ng, which is further enhanced when the concentration of the DN-TonEBP is increased to 300 ng (Fig. 3A). On the other hand, overexpression of TonEBP under isotonic conditions, using pFLAG-TonEBP vector, results in a dose-dependent increase in AQP2 (Fig. 3C) and TauT (Fig. 3D) promoter activities. We used MEFs derived from TonEBP/NFAT5-null and wildtype mice to further validate the inductive effect of TonEBP on AQP2. Figure 3E shows that hypertonicity results in a small but significant increase in AQP2 promoter activity in wildtype cells. Interestingly, under hypertonic conditions, there is an ∼50% decrease in AQP2 promoter activity in TonEBP-null cells. As expected, hypertonicity causes a significant increase (20- to 25-fold) in TauT reporter activation in wildtype cells, whereas in null MEFs, activity did not change significantly (Fig. 3F).
FIG. 3.
TonEBP regulation of AQP2 promoter activity. AQP2 (A) or TauT (B) reporter constructs along with DN-TonEBP, or empty backbone FLAG-CMV2, were transfected into NP cells, and luciferase activity was measured. Expression of DN-TonEBP resulted in a complete suppression of hypertonicity-mediated induction in AQP2 and TauT promoter activity. When pTonEBP was co-expressed with AQP2 (C) or TauT (D) under isotonic conditions, there was a significant increase in activity of both the reporters in NP cells. Note, the activity of mutant AQP2 reporter was unaffected by TonEBP. NFAT5-null and wildtype MEFs were transfected with AQP2 (E) or TauT (F) reporter and cultured under isotonic or hypertonic conditions (500 mosmol/kg). Wildtype cells evidenced an increase in activity of both the reporters under hypertonic conditions. In contrast, in hypertonic media, null MEFs showed a significant suppression of AQP2 reporter activity, whereas TauT activity remained unchanged. Values shown are the mean ± SD of three independent experiments performed in triplicate, *p < 0.05.
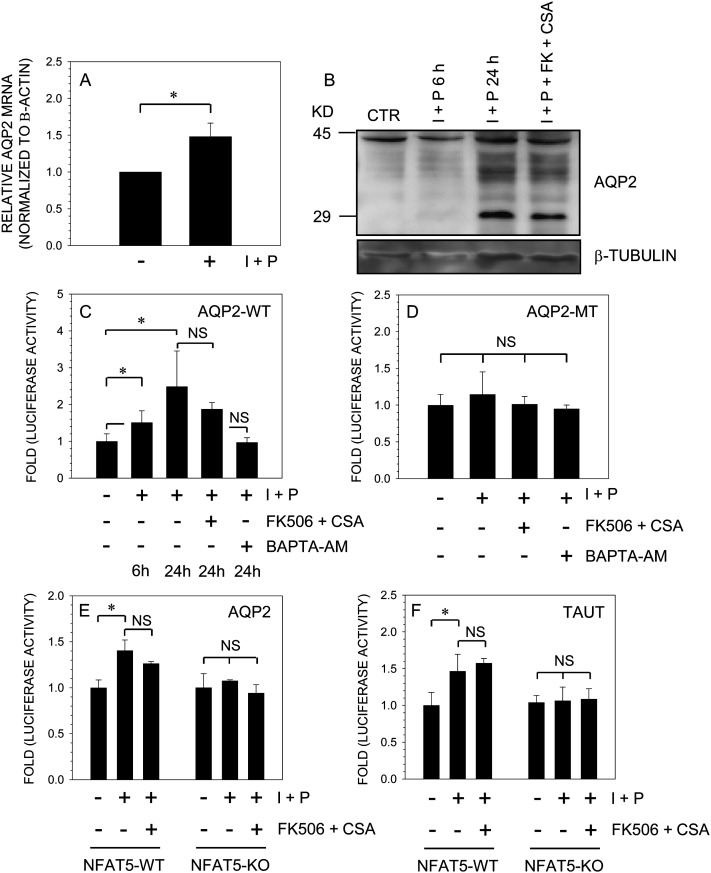
To ascertain whether calcineurin signaling plays role in AQP2 expression in nucleus pulposus cells, we treated cells with the calcium ionophore, ionomycin, together with PMA. This chemical cocktail activates calcineurin-mediated signaling. Figures 4A and 4B show that treatment with ionomycin results in increased AQP2 mRNA (Fig. 4A) and protein (Fig. 4B) expression. Surprisingly, inhibitors of calcineurin signaling, FK506 and cyclosporine A (CsA), did not diminish AQP2 protein levels (Fig. 4B). To confirm these findings, we transfected nucleus pulposus cells with wildtype or mutant AQP2 reporters and measured their activity after ionomycin treatment. Figures 4C and 4D show that ionomycin treatment results in an increase in wildtype (Fig. 4C) but not mutant (Fig. 4D) AQP2 promoter activity. Inclusion of FK506 and CsA did not block ionomycin mediated induction in AQP2 promoter activity. However, treatment with the calcium chelator BAPTA-AM completely suppressed inomycin-mediated induction in AQP2 reporter activity. To further validate these findings, we used NFAT5-null and wildtype MEFs and measured activities of AQP2 and TauT reporters after ionomycin treatment. Figure 4 shows that ionomycin treatment leads to an increase in both AQP2 (Fig. 4E) and TauT (Fig. 4F) reporter activities. Again, inclusion of FK506 and CsA did not affect promoter induction in wildtype cells. In contrast, in NFAT5-null cells, ionomycin did not in increase either AQP2 (Fig. 4E) or TauT (Fig. 4F) reporter activities.
FIG. 4.
Effect of calcium ions on AQP2 expression. NP cells were treated with the calcium ionophore, ionomycin (I; 1 μM), and PMA (P; 100 ng), and AQP2 expression was measured. (A) Ionomycin treatment resulted in small but significant increase in AQP2 mRNA expression. (B) After treatment, AQP2 levels were measured by Western blot analysis. Note, the increase in newly synthesized AQP2 protein (29 kDa) after ionomycin treatment. Addition of calcineurin inhibitors, FK506 (10 ng/ml) and cyclosporine A (CsA; 1 μg/ml), did not suppress synthesis of AQP2 protein. (C and D) NP cells were transfected with wildtype (C) or mutant (D) AQP2 reporter and treated with ionomycin and PMA with or without FK506 and CsA or BAPTA-AM (10 μM). Note treatment with ionomycin increased the activity of the wildtype (WT) but not the mutant (MT) AQP2 reporter. FK506 and CsA did not inhibit ionomycin-mediated increase in AQP2-WT promoter activity, whereas BAPTA-AM completely blocked the induction. The activity of AQP2 (E) or TauT (F) reporters was measured in NFAT5 wildtype (Wt) or knockout (KO) MEFs after ionomycin treatment. In contrast to the WT cells, the KO cells did not exhibit an increase in activity of both promoters after ionomycin treatment. FK506 and CsA did not block the inductive effect of ionomycin on either of the reporters in NFAT5-WT cells. Values shown are the mean ± SD of three independent experiments; *p < 0.05.
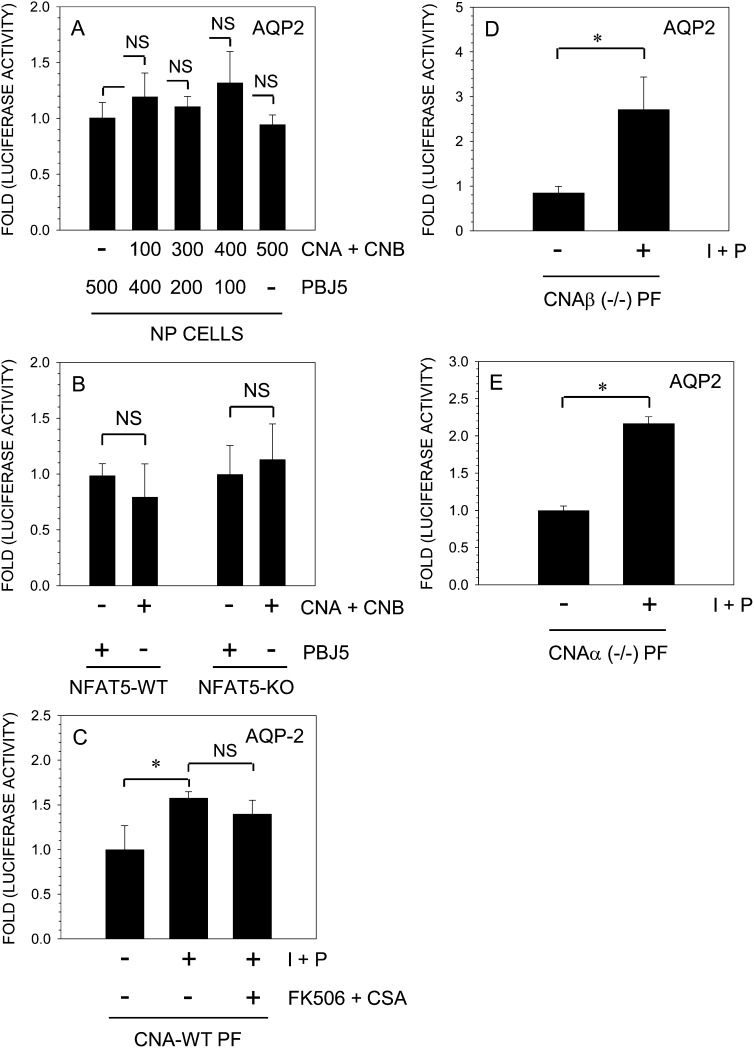
To determine the role of calcineurin signaling in AQP2 expression, we co-transfected rat nucleus pulposus cells with plasmids expressing CnA and calcineurin B (CnB) along with an AQP2 reporter. Figure 5A shows that calcineurin overexpression had no measurable inductive effect on AQP2 promoter activity. We measured AQP2 reporter activity in NFAT5 wildtype and null cells after calcineurin overexpression. Again, calcineurin did not induce AQP2 reporter activity. To further evaluate whether calcineurin signaling was involved, we used primary fibroblasts derived from CnAα-null, CnAβ-null, and wildtype mice. Treatment of wildtype cells with ionomycin results in a small but significant increase in AQP2 reporter activity; again, FK506 and CsA fail to block this activation (Fig. 5C). Interestingly, in both CnAα-null and CnAβ-null fibroblasts, ionomycin treatment results in pronounced activation (2- to 3-fold) of the AQP2 reporter.
FIG. 5.
Calcineurin signaling does not participate in regulation of AQP2 promoter activity in NP cells. The AQP2 reporter along with calcineurin A/B constructs or empty vector pBJ5 was transfected into NP cells (A) or NFAT5-null and wildtype MEFs (B), and luciferase activity was measured. The co-expression of calcineurin subunits did not increase AQP2 reporter activity in transfected cells. (C–E) Primary fibroblasts (PFs) derived from calcineurin wildtype (WT) or CnAα- or CnAβ-null (−/−) mice were transfected with AQP2 reporter and treated with ionomycin and PMA with or without FK506 and CsA. Note, ionomycin significantly increased AQP2 reporter activity in both the CnAα- and CnAβ-null cells; a relatively small inductive effect was seen in wildtype cells that remained constant after addition of calcineurin inhibitors, FK506 and CsA. Values shown are the mean ± SD of three independent experiments performed in triplicate; *p < 0.05
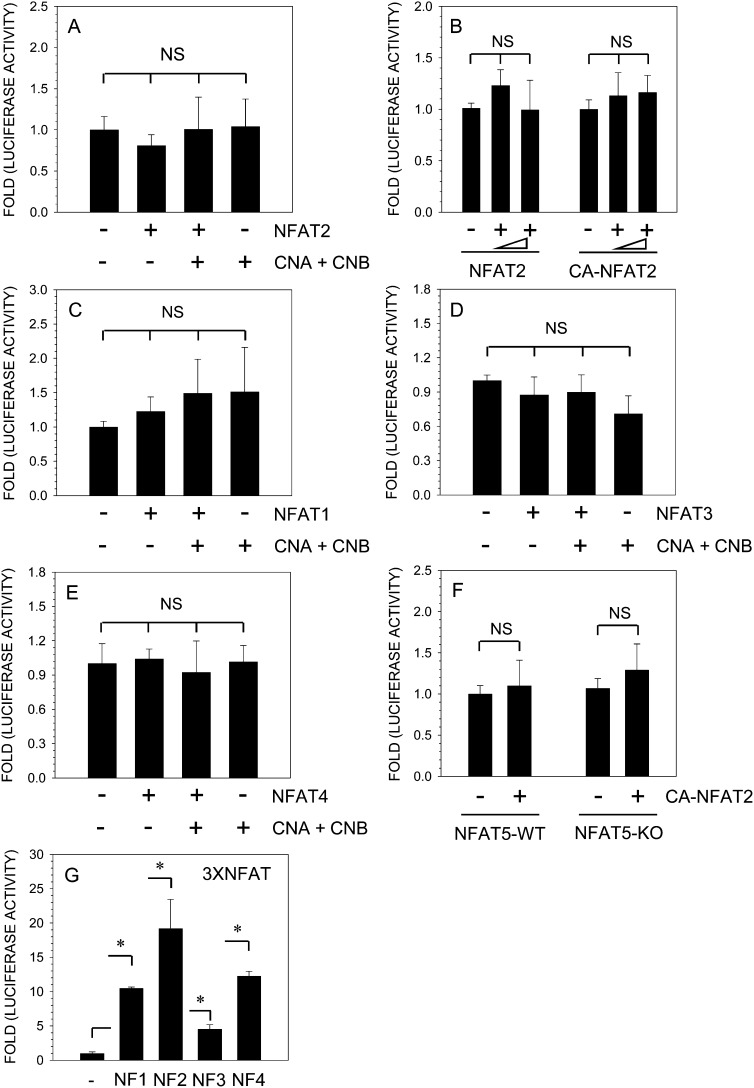
To study the relationship between AQP2 and NFAT signaling, we transfected nucleus pulposus cells with plasmids encoding NFAT1–4 and measured the activity of the AQP2 reporter. Figure 6A shows that co-expression of NFAT2, calcineurin, or both has no effect on AQP2 reporter activity. To confirm this observation, we performed a dose-response study using both NFAT2 and CA-NFAT2 expression vectors. Increasing concentration of either NFAT2 or CA-NFAT2 has no effect on AQP2 reporter activity in nucleus pulposus cells (Fig. 6B). Similarly, co-expression of NFAT1 (Fig. 6C), NFAT3 (Fig. 6D), or NFAT4 (Fig. 6E) with or without calcineurin plasmids has minimal effect on AQP2 reporter activation in nucleus pulposus cells. In a parallel experiment, we used NFAT5/TonEBP-null and wildtype MEFs and measured AQP2 reporter activity after transfection with CA-NFAT2. In both wildtype and null NFAT5 cells, CA-NFAT2 failed to increase AQP2 promoter activity (Fig. 6F). To confirm that transfected NFAT plasmids expressed functional proteins in nucleus pulposus cells, we measured activation of a NFAT responsive 3xNFAT reporter. Figure 6G shows that there is significant activation of 3xNFAT luciferase reporter when co-transfected with NFAT1 or CA-NFAT2, NFAT3, or NFAT4 expression plasmids.
FIG. 6.
AQP2 promoter activity is independent of NFAT signaling in NP cells. NP cells were transfected with NFAT vectors (NFAT2, -1, -3, -4) and/or CnA and CnB along with AQP2 reporter. (A) NFAT2 or calcineurin alone or together did not affect AQP2 reporter activity. (B) AQP2 reporter activity was not induced when co-transfected with increasing dose (200–400 ng) of either NFAT2 or CA-NFAT2 expression plasmid. NFAT1 (C), NFAT3 (D), or NFAT4 (E) alone or when added with calcineurin did not significantly change the AQP2 reporter activity in NP cells. (F) NFAT5 wildtype (WT) and null (KO) cells were transfected with AQP2 reporter with constitutively active (CA)-NFAT2 plasmid (100 ng). Note, AQP2 reporter activity in both NFAT5-WT and KO cells did not change significantly in the presence of CA-NFAT2. (G) NP cells were transfected with 3xNFAT luciferase construct with or without NFAT1 or CA-NFAT2 or NFAT3 or NFAT4, and reporter activity was measured. Co-expression of NFAT1–4 resulted in a significant increase in activity of 3xNFAT reporter plasmid indicating functionality of expressed proteins. Values shown are the mean ± SD of three independent experiments; *p < 0.05, ns = nonsignificant.
DISCUSSION
The experiments described in this study showed for the first time that AQP2, a tonicity-sensitive water channel protein, was expressed by nucleus pulposus cells that populate the hydrodynamically stressed, hyperosmolar microenvironment of the intervertebral disc. Moreover, AQP2 expression was regulated by TonEBP, a transcription factor that we have shown to promote aggrecan gene expression.(6) In addition, our studies showed that, aside from osmolarity, AQP2 activity in nucleus pulposus cells is dependent on intracellular calcium levels but independent of calcineurin–NFAT signaling pathways. This observation is particularly relevant to disc cell function, because calcium signaling is closely linked to the transduction of applied biomechanical forces. Accordingly, expression of TonEBP permits nucleus pulposus cells to autoregulate the osmotic environment by controlling water transport while at the same time permitting cells to accommodate to mechanical loading, a functional characteristic of the disc microenvironment.
Building on earlier observations that the cells in the nucleus pulposus exist in a unique microenvironment, we evaluated AQP expression in the discal tissues. At the mRNA and protein level, there was a robust expression of AQP2 in nucleus pulposus cells of both the neonatal and mature rat discs; the protein was also expressed by cells of the annulus fibrosus. This observation was not unexpected because the annulus, like the nucleus, is rich in proteoglycans. Moreover, because the annulus is under tension and mechanically stressed,(4) AQP2 would serve to enhance water transport in these cells. Surprisingly, we found that this protein was strongly expressed in degenerate human disc tissue. Possibly as a survival mechanism, disc cells may respond to the loss of matrix glycans that accompany tissue degradation by promoting their ability to transport water from the extracellular matrix to the cell interior. That AQP2 was expressed by the nucleus pulposus was in contrast to an earlier immunohistological study where it was reported that this protein was not expressed in the human disc.(22) Although this negative result may have been caused by an unreactive antibody, this study was able to show the expression of this protein in native rat tissue, human degenerative samples, and cells in culture using a plethora of analytical techniques. Moreover, microarray analysis by two independent groups have confirmed AQP2 mRNA expression in human nucleus pulposus and annulus fibrosus tissue (Dr. Sibylle Grad, AO Research Institute, and Dr. Helen Gruber, Carolinas Health Center, personal communications).
Gain and loss of function experiments were performed to learn whether hyperosmolarity induced transcriptional activation of AQP2 reporter activity and whether this activation was regulated by TonEBP. In hypertonic medium, suppression of TonEBP activity blocked induction of AQP2; pTonEBP enhanced AQP2 reporter expression under isotonic conditions. We used MEFs derived from TonEBP/NFAT5 wildtype and null mice to confirm these findings because of similarities in the response by nucleus pulposus cells and TonEBP wildtype MEFs to hypertonic stimulation. In hypertonic media, TonEBP-null MEFS failed to induce AQP2 promoter activity. Together, the results of these functional studies indicated that, in addition to regulating the taurine transporter (TauT), other osmolytes, and HSP-70,(6,7,14,23) TonEBP serves to adapt nucleus pulposus cells to the hypertonic conditions of the disc by controlling the expression of AQP2.
The mechanism of activation of TonEBP is not completely understood, especially whether it is mediated by direct phosphorylation of the protein.(13) There is also some evidence in T cells and kidney cells to indicate that regulation may be mediated by a phosphatase, calcineurin, which is activated by calcium ions.(24,12) The results of the studies described herein suggest that this mechanism may be more cell type specific. Treatment of nucleus pulposus cells with cyclosporine and FK506, agents that inhibit calcineurin signaling, failed to change the promoter activities of the TonEBP target gene TauT. Further support of the notion that calcineurin signaling was not needed for TonEBP-dependent promoter activation was from studies performed on TonEBP/NFAT5-null MEFs. We noted that, after ionomycin treatment, the null cells failed to induce AQP2 and TauT reporter activity. Overexpression of calcineurin in NFAT5-null MEFs and nucleus pulposus cells did not elevate AQP2 reporter activity. Moreover, ionomycin treatment of primary fibroblasts derived from calcineurin (CnAα and CnAβ) null mice induced AQP2 reporter activity. Because of the similar response of CnA wildtype fibroblasts and nucleus pulposus cells to ionomycin treatment, fibroblasts from CnA-null mice were chosen as controls to monitor AQP2 promoter activity. These studies indicated that AQP2 activity was not dependent on calcineurin; in contrast, because ionomycin preferentially raises the intracellular calcium ion concentration, studies using BAPTA-AM, a calcium chelator, supported the notion that stimulation of AQP2 reporter activity was caused by a rise in the intracellular activity of this ion. From a functional viewpoint, the critical role of calcium ions in this transduction system is of considerable importance. Mechanical shear and loading induce calcium transients in cells of many skeletal tissues including the intervertebral disc.(5) Thus, processes that cause an increase in intracellular calcium level may well provide one mechanism by which mechanical forces influence not just membrane deformation but also signal changes in local osmotic conditions.
Whereas we have shown that ionomycin profoundly influences TonEBP-dependent promoter activity, we considered the possibility that other members of the NFAT family may be needed for AQP2 promoter activity. Results of one recent study suggested that NFATc1 or NFAT2 serves as a regulator of AQP2 transcription.(12) It is well documented that calcineurin-mediated dephosphorylation of SP motifs results in the nuclear import of NFAT and stimulation of transcription.(25) However, an increasing number of studies also suggest that inducible phosphorylation of the transactivation domain (TAD) of NFAT and other mechanisms may regulate its activity, lending support to a calcineurin-independent pathway.(26–29) To address this possibility, we overexpressed individual NFATs and determined AQP2 reporter activity. In line with previous studies,(12) we found that NFAT1, -3, and -4 did not increase AQP2 promoter activity. A lack of AQP2 induction after co-expression of constitutively active NFAT2 provided evidence that, unlike the kidney, AQP2 promoter activity is independent of NFAT2 activation in nucleus pulposus cells. That expressed NFATs were functional was confirmed by measuring activation of 3xNFAT luciferase reporter that contains three NFAT binding sites upstream of minimal interleukin (IL)-2 promoter. Based on these findings, we conclude that NFAT5/TonEBP is the major regulator of AQP2 promoter activity in cells of the nucleus pulposus.
In summary, cells of the nucleus pulposus are adapted to function in an environmental niche that has a limited oxygen supply and a high osmotic pressure. We showed that NFAT5/TonEBP regulates a critical water transport gene, AQP2. This finding, together with the observation that TonEBP governs the expression of osmolytes and osmolyte transporters, provides a new insight into mechanisms by which this transcription factor regulates the osmolarity of the nucleus pulposus cells. Analysis of other factors influencing AQP2 expression indicates that promoter activity is influenced by shifts in calcium levels. Because calcium flux reflects a change in applied stress, the possibility exists that NFAT5/TonEBP modulates not just water balance in the disc but also accommodates functional biomechanical forces.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health Grants AR050087 and AR055655. We thank Drs. Sibylle Grad and Helen Gruber for sharing their unpublished data.
Footnotes
The authors state that they have no conflicts of interest.
REFERENCES
- 1.Maroudas A, Muir H, Wingham J. The correlation of fixed negative charge with glycosaminoglycan content of human articular cartilage. Biochim Biophys Acta. 1969;177:492–500. doi: 10.1016/0304-4165(69)90311-0. [DOI] [PubMed] [Google Scholar]
- 2.Maroudas A. Distribution and diffusion of solutes in articular cartilage. Biophys J. 1970;10:365–379. doi: 10.1016/S0006-3495(70)86307-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Urban JP, Holm S, Maroudas A. Diffusion of small solutes into the intervertebral disc: As in vivo study. Biorheology. 1978;15:203–221. doi: 10.3233/bir-1978-153-409. [DOI] [PubMed] [Google Scholar]
- 4.Kolditz D, Gowin R. Water and electrolyte content of human intervertebral discs under variable load. Spine. 1985;10:69–71. doi: 10.1097/00007632-198501000-00011. [DOI] [PubMed] [Google Scholar]
- 5.Pritchard S, Erickson GR, Guilak F. Hyperosmotically induced volume change and calcium signaling in intervertebral disk cells: The role of the actin cytoskeleton. Biophys J. 2002;83:2502–2510. doi: 10.1016/S0006-3495(02)75261-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsai TT, Danielson KG, Guttapalli A, Oguz E, Albert TJ, Shapiro IM, Risbud MV. TonEBP/OREBP is a regulator of nucleus pulposus cell function and survival in the intervertebral disc. J Biol Chem. 2006;281:25416–25424. doi: 10.1074/jbc.M601969200. [DOI] [PubMed] [Google Scholar]
- 7.Tsai TT, Guttapalli A, Agrawal A, Albert TJ, Shapiro IM, Risbud MV. MEK/ERK signaling controls osmoregulation of nucleus pulposus cells of the intervertebral disc by transactivation of TonEBP/OREBP. J Bone Miner Res. 2007;22:965–974. doi: 10.1359/jbmr.070322. [DOI] [PubMed] [Google Scholar]
- 8.Hall AC, Bush PG. The role of a swelling-activated taurine transport pathway in the regulation of articular chondrocyte volume. Pflugers Arch. 2001;442:771–781. doi: 10.1007/s004240100601. [DOI] [PubMed] [Google Scholar]
- 9.Fu D, Lu M. The structural basis of water permeation and proton exclusion in aquaporins. Mol Membr Biol. 2007;24:366–374. doi: 10.1080/09687680701446965. [DOI] [PubMed] [Google Scholar]
- 10.Verkman AS. Roles of aquaporins in kidney revealed by transgenic mice. Semin Nephrol. 2006;26:200–208. doi: 10.1016/j.semnephrol.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Hasler U, Jeon US, Kim JA, Mordasini D, Kwon HM, Féraille E, Martin PY. Tonicity-responsive enhancer binding protein is an essential regulator of aquaporin-2 expression in renal collecting duct principal cells. J Am Soc Nephrol. 2006;17:1521–1531. doi: 10.1681/ASN.2005121317. [DOI] [PubMed] [Google Scholar]
- 12.Li SZ, McDill BW, Kovach PA, Ding L, Go WY, Ho SN, Chen F. Calcineurin-NFATc signaling pathway regulates AQP2 expression in response to calcium signals and osmotic stress. Am J Physiol Cell Physiol. 2007;292:C1606–C1616. doi: 10.1152/ajpcell.00588.2005. [DOI] [PubMed] [Google Scholar]
- 13.Jeon US, Kim JA, Sheen MR, Kwon HM. How tonicity regulates genes: Story of TonEBP transcriptional activator. Acta Physiol (Oxf) 2006;187:241–247. doi: 10.1111/j.1748-1716.2006.01551.x. [DOI] [PubMed] [Google Scholar]
- 14.Ito T, Fujio Y, Hirata M, Takatani T, Matsuda T, Muraoka S, Takahashi K, Azuma J. Expression of taurine transporter is regulated through the TonE (tonicity-responsive element)/TonEBP (TonE-binding protein) pathway and contributes to cytoprotection in HepG2 cells. Biochem J. 2004;382:177–182. doi: 10.1042/BJ20031838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lam AK, Ko BC, Tam S, Morris R, Yang JY, Chung SK, Chung SS. Osmotic response element-binding protein (OREBP) is an essential regulator of the urine concentrating mechanism. J Biol Chem. 2004;279:48048–48054. doi: 10.1074/jbc.M407224200. [DOI] [PubMed] [Google Scholar]
- 16.Monticelli S, Rao A. NFAT1 and NFAT2 are positive regulators of IL-4 gene transcription. Eur J Immunol. 2002;32:2971–2978. doi: 10.1002/1521-4141(2002010)32:10<2971::AID-IMMU2971>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 17.Ichida M, Finkel T. Ras regulates NFAT3 activity in cardiac myocytes. J Biol Chem. 2001;276:3524–3530. doi: 10.1074/jbc.M004275200. [DOI] [PubMed] [Google Scholar]
- 18.Clipstone NA, Crabtree GR. Identification of calcineurin as a key signalling enzyme in T-lymphocyte activation. Nature. 1992;357:695–697. doi: 10.1038/357695a0. [DOI] [PubMed] [Google Scholar]
- 19.Risbud MV, Guttapalli A, Stokes DG, Hawkins D, Danielson KG, Schaer TP, Albert TJ, Shapiro IM. Nucleus pulposus cells express HIF-1alpha under normoxic culture conditions: A metabolic adaptation to the intervertebral disc microenvironment. J Cell Biochem. 2006;98:152–159. doi: 10.1002/jcb.20765. [DOI] [PubMed] [Google Scholar]
- 20.Hilibrand AS, Carlson GD, Palumbo MA, Jones PK, Bohlman HH. Radiculopathy and myelopathy at segments adjacent to the site of a previous anterior cervical arthrodesis. J Bone Joint Surg Am. 1999;81:519–528. doi: 10.2106/00004623-199904000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Zeng Y, Danielson KG, Albert TJ, Shapiro IM, Risbud MV. HIF-1 alpha is a regulator of galectin-3 expression in the intervertebral disc. J Bone Miner Res. 2007;22:1851–1861. doi: 10.1359/jbmr.070620. [DOI] [PubMed] [Google Scholar]
- 22.Richardson SM, Knowles R, Marples D, Hoyland JA, Mobasheri A. Aquaporin expression in the human intervertebral disc. J Mol Histol. 2008;39:303–309. doi: 10.1007/s10735-008-9166-1. [DOI] [PubMed] [Google Scholar]
- 23.Woo SK, Lee SD, Na KY, Park WK, Kwon HM. TonEBP/NFAT5 stimulates transcription of HSP70 in response to hypertonicity. Mol Cell Biol. 2002;22:5753–5760. doi: 10.1128/MCB.22.16.5753-5760.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trama J, Lu Q, Hawley RG, Ho SN. The NFAT-related protein NFATL1 (TonEBP/NFAT5) is induced upon T cell activation in a calcineurin-dependent manner. J Immunol. 2000;165:4884–4894. doi: 10.4049/jimmunol.165.9.4884. [DOI] [PubMed] [Google Scholar]
- 25.Hogan PG, Chen L, Nardone J, Rao A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 2003;17:2205–2232. doi: 10.1101/gad.1102703. [DOI] [PubMed] [Google Scholar]
- 26.Gómez-Casero E, San-Antonio B, Iñiguez MA, Fresno M. Cot/Tpl2 and PKCzeta cooperate in the regulation of the transcriptional activity of NFATc2 through the phosphorylation of its amino-terminal domain. Cell Signal. 2007;19:1652–1661. doi: 10.1016/j.cellsig.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 27.Ortega-Pérez I, Cano E, Were F, Villar M, Vázquez J, Redondo JM. c-Jun N-terminal kinase (JNK) positively regulates NFATc2 transactivation through phosphorylation within the N-terminal regulatory domain. J Biol Chem. 2005;280:20867–20878. doi: 10.1074/jbc.M501898200. [DOI] [PubMed] [Google Scholar]
- 28.Kuroda Y, Hisatsune C, Nakamura T, Matsuo K, Mikoshiba K. Osteoblasts induce Ca2+ oscillation-independent NFATc1 activation during osteoclastogenesis. Proc Natl Acad Sci USA. 2008;105:8643–8648. doi: 10.1073/pnas.0800642105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shen T, Liu Y, Cseresnyés Z, Hawkins A, Randall WR, Schneider MF. Activity- and calcineurin-independent nuclear shuttling of NFATc1, but not NFATc3, in adult skeletal muscle fibers. Mol Biol Cell. 2006;17:1570–1582. doi: 10.1091/mbc.E05-08-0780. [DOI] [PMC free article] [PubMed] [Google Scholar]