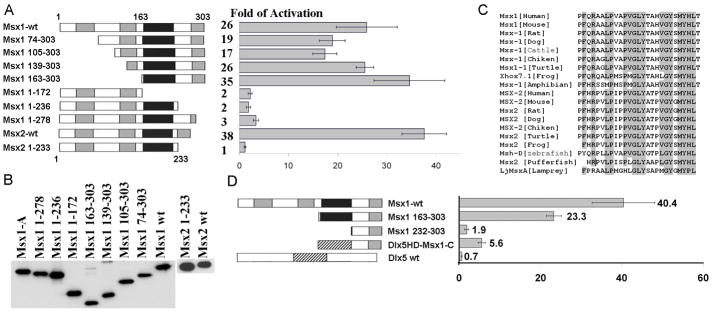
FIG. 2. The transcriptional activation activity resides in the C-terminal domain of Msx proteins.
(A) Schematic representations of the full-length and deletion mutants of Msx1 and Msx2. Areas that shaded gray represent Msx conserved regions and areas that shaded black correspond to the homeodomain. Removal of the C-terminal domain in both Msx1 and Msx2 protein leaded to a significant reduction in reporter activity. (B) Msx mutants that were transfected into C2C12 cells were stably translated. Flag-tagged Msx1 and Msx2 wild type and mutants proteins were detected on western blots using the anti-Flag antibody. (C) The C-terminal domains are highly conserved among Msx1 and Msx2 proteins in vertebrates. Identical amino acids were shaded in gray. (D) Schematic of replacement of Msx homeodomain (black) with Dlx5 homeodomain (striped rectangle). Removal of the homeodomain (Msx1 232-303) resulted in a significant reduction in the magnitude of transcriptional stimulation in comparison to Msx1 163-303 which contains the homeodomain. Replacement of the Msx1 homeodomain with the Dlx5 homeodomain restored the activation potency. 100 ng of Hsp205-LacZ reporters with 300 ng Msx1 mutant derivatives, Msx1-Dlx5HD or pIRES-hrGFP-1a were co-transfected into C2C12 cells. Transfections were performed in triplicates, and the standard error is shown.