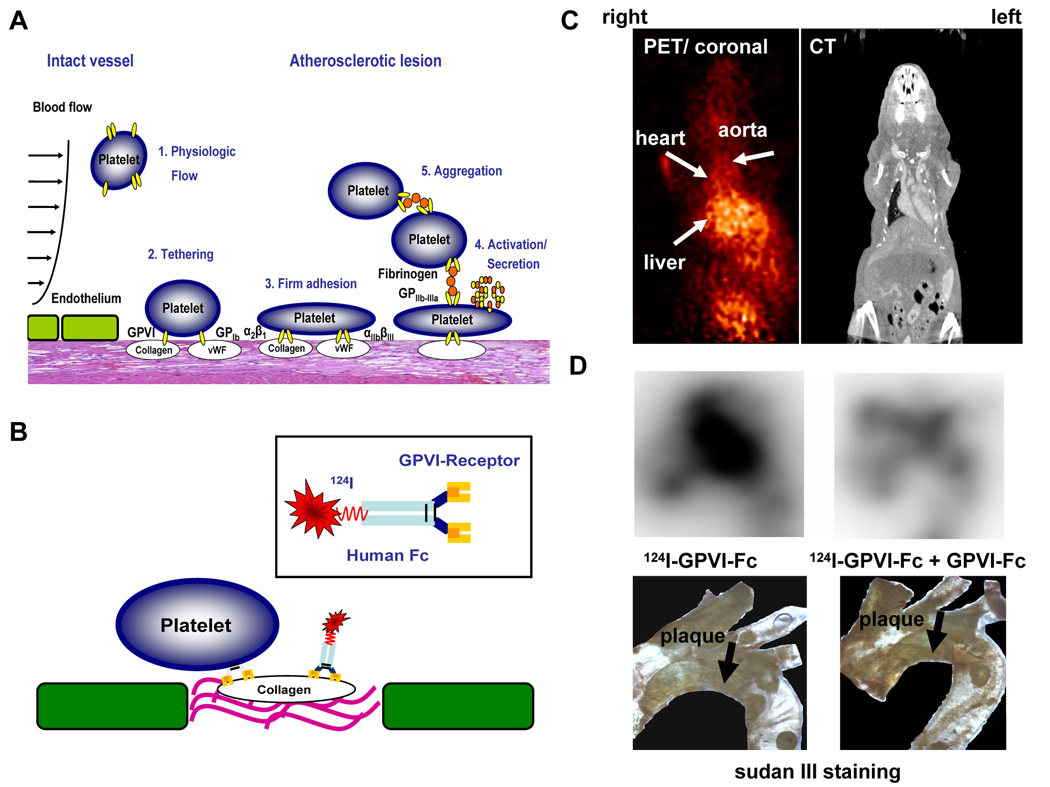
Fig 5. Detection of vulnerable, thrombogenic plaques by radiolabeled platelet GPVI.
(A) Pathophysiology of platelet adhesion, secretion and thrombus formation at sites of injured vascular endothelium with exposed extracellular matrix. (1.) Within the intact vessel, platelets do not adhere to the endothelial monolayer under physiological conditions. (2.) At site of atherosclerotic lesions, subendothelial proteins like von Willebrand factor (vWF) and collagen are exposed to blood flow. Platelet adhesion receptors GPIb and GPVI mediate tethering of platelets. (3.) After activation of the integrins α2β1 (collagen receptor) and αIIbβ3 (fibrinogen receptor), platelets firmly adhere via interaction of these receptors with extracellular matrix proteins. (4.) Subsequently, platelets get activated and secrete distinct mediators resulting in (5.) platelet aggregation via fibrinogen bridges between two αIIbβ3 receptors and thrombus formation. (B) A soluble dimeric form of human platelet GPVI conjugated to an Fc-fragment was used, which was radiolabeled with 124I. GPVI is essential to establish the first interaction of platelets with an exposed collagen surface. Therefore, we made use of this natural mechanism to detect thrombogenic, and thus, vulnerable plaques. (C, D) ApoE −/− mice were analyzed with 124IGPVI-Fc using an animal microPET scanner (MicroPET Focus 120, Siemens, Germany) (left panel). Images were aquired 24 hours after administration of the tracer and imaging time was 20 minutes. PET images were correlated with CT data (right panel) to verify anatomical structures. (C) shows transverse sections of these experiments. (D) Ex vivo nuclear imaging was performed to evaluate tracer activity in the aortic arch (upper panels). The lower panels show the specimen after staining with sudan III. Activity of 124IGPVI-Fc correlated well with plaque extension (left panel). The signal could be nearly abolished, when non-labeled GPVI-Fc was injected prior to application of 124IGPVI-Fc. (right panel).