Abstract
The mononuclear copper enzyme, galactose oxidase, has been investigated under steady-state conditions via O2-consumption assays using 1-O-methyl-α-D-galactopyranoside as the sugar substrate to produce an aldehyde at the C-6 position. The rate-determining step of the oxidative half-reaction was probed through the measurement of substrate and solvent deuterium and O-18 isotope effects on kcat/Km(O2). The reaction conforms to a ping-pong mechanism with the kinetic parameters for the reductive half, kcat/Km(S) = 8.3 × 103 M−1 s−1 at 10°C and pH 7.0, comparing favorably to literature values. The oxidative half-reaction yielded a value of kcat/Km(O2) = 2.5 × 106 M−1 s−1. A substrate deuterium isotope effect of 32 was measured for the kcat/Km(S), while a smaller, but significant value of 1.6-1.9 was observed on kcat/Km(O2). O-18 isotope effects of 1.0185 with either protiated or deuterated sugar, together with the absence of any solvent isotope effect, lead to the conclusion that hydrogen atom transfer from reduced cofactor to a Cu(II)-superoxo intermediate is fully rate-determining for kcat/Km(O2). The measured O-18 isotope effects provide corroborative evidence for the reactive superoxo species in the dopamine β-monooxygenase/peptidylglycine α-hydroxylating monooxygenase family, as well as providing a frame of reference for copper-superoxo reactivity. The combination of solvent and substrate deuterium isotope effects rules out solvent deuterium exchange into reduced enzyme as the origin of the relatively small substrate deuterium isotope effect on kcat/Km(O2). These data indicate fundamental differences in the hydrogen transfer step from the carbon of substrate vs. the oxygen of reduced cofactor during the reductive and oxidative half reactions of galactose oxidase.
Keywords: Copper superoxo reactivity, galactose oxidase, oxidative half reaction, deuterium, O-18 isotope effects
1. Introduction
Galactose oxidase (GAOX) has been the subject of extensive study over a period of several decades. Interest in this extracellular copper enzyme was initially fueled by the mystery surrounding the prospect of a two-electron oxidation/reduction mediated by a single copper atom. Recent interest, however, can be attributed to the discovery of the enzyme's protein-derived radical cofactor (TyrCys) formed via cross-linking of an active site tyrosine to cysteine.1,2 GAOX catalyzes the oxidation of a broad range of primary alcohols to their corresponding aldehyde. Stopped flow studies have demonstrated that the net reaction can be separated into two half-reactions,3,4 the first of which involves substrate oxidation and reduction of the enzyme to yield Cu(I)-tyrosinate, eq. (1):
| (1) |
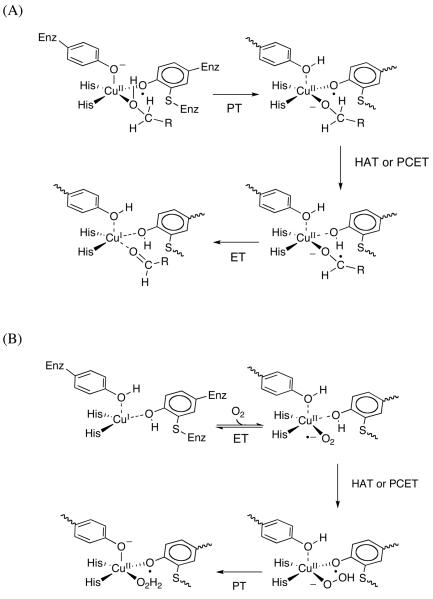
This process can be further broken down into three steps: proton transfer from substrate to an active site base, hydrogen atom transfer to the cofactor-based radical center, and electron transfer to the Cu(I), Scheme 1A. A large kinetic isotope effect has been observed on the rate of sugar oxidation, ascribed to H-atom tunneling from substrate to cofactor.3
Scheme 1.
Working Chemical Mechanisms for GAOX: (A) Reductive Half Reaction (in which bound substrate is converted to bound product); and (B) Oxidative Half-Reactions of GAOX (in which free O2 is converted to bound H2O2).
Considerably less is known about the oxidative process resulting in production of hydrogen peroxide from dioxygen and regeneration of the radical cofactor and Cu(II), eq. (2):
| (2) |
Analogous to the reductive half reaction, a three-step mechanism can be proposed for the recycling of reduced enzyme (Scheme 1B). In the first step, electron transfer from Cu(I) to coordinated oxygen yields metal-bound superoxide. In a second step, metal-bound superoxide abstracts a hydrogen atom from the phenolic hydroxyl group of the TyrCys cofactor to produce hydroperoxide and TyrCys•. Finally, a proton is transferred from the axial tyrosine to metal-bound hydroperoxide to produce hydrogen peroxide and fully re-oxidized enzyme.
Historically, the superoxide ion had been considered too “sluggish” an oxidant, relative to peroxide and hydroxyl radical, for efficient H-atom abstraction. In this context, there has been lengthy debate over the possible activated oxygen intermediates utilized by the copper enzymes, dopamine β–monooxygenase (DβM) and peptidylglycine α-hydroxylating monooxygenase (PHM), during substrate hydroxylation.5-8 Most recently, these enzymes have been concluded to employ a mono-nuclear cupric superoxide ion, based on extensive steady-state kinetics data that include deuterium and O-18 isotope effects and product analyses that implicate full coupling between dioxygen and substrate uptake.9,10 DFT calculations have provided strong support for the involvement of a metal superoxide complex as the hydroxylating agent,11 while X-ray studies of PHM, characterized in the presence of a slow substrate and O2, indicate a bound oxygen species that may well be superoxide ion.12 As introduced above, consideration of possible mechanisms for reaction of reduced GAOX with O2 points toward initial formation of a superoxide intermediate via electron transfer from Cu(I) to dioxygen. Further, the fact that hydrogen peroxide is the product of the galactose oxidase reaction makes superoxide the only reasonable mediator of H-atom abstraction from reduced cofactor. We, thus, present GAOX as an ideal system in which to investigate the properties of superoxide reactivity at enzymatic mononuclear copper centers.
Steady-state kinetics that include substrate and solvent deuterium and O-18 kinetic isotope effects can provide remarkable insight into the rate-controlling step(s) in O2-utilizing enzymes as well as, in selected cases, the nature of reactive oxygen intermediates. Comparison of the present kinetic results for GAOX to data for PHM and DβM provides corroborative evidence in support of the proposed superoxo- intermediate in the latter systems. In particular, the size of the O-18 KIE provides a frame of reference for the reactivity of Cu(II)(O2·−) in both oxidase and oxygenase-type enzymes. These studies also allow comparison of the impact of substrate deuteration on the properties of alcohol oxidation (C-H abstraction) vs. O2 reduction (O-H activation) within a single enzyme active site.
2. Experimental
Materials and Methods
1-O-methyl-α-D-galactopyranoside (H2-OMeGal), catalase, potassium ferricyanide, 3-methoxybenzyl alcohol, and D2O were purchased from Sigma-Aldrich and used without further purification. The 1-O-methyl-[6,6-2H2]-α-D-galactopyranoside (D2-OMeGal) substrate was synthesized according to the literature.3 Ferricyanide stock solutions were prepared fresh daily. GAOX was found to be highly sensitive to contaminants on the surface of glass- and plasticware. Vessels used during the fungal growth and enzyme purification steps were rinsed three times with de-ionized water prior to use. GAOX was stored at −80 °C in DNase/RNase- and pyrogen-free microcentrifuge tubes (USA Scientific). Activated GAOX was stored in glass vials that had been washed with hexanes, 6 M nitric acid, de-ionized water, and methanol, then dried in an oven. The cuvettes for spectroscopic assays were rinsed with 1 M nitric acid, de-ionized water and methanol, then thoroughly dried.
Purification and Isolation of GAOX
GAOX was isolated from the growth medium of the fungus, D. dendroides, (spp. Fusarium, ATCC 46032) and purified according to the literature procedure of Tressel and Kosman.13 Exceptions to this protocol are as follows: catalase (10 mg/L) was added to the dialysis buffer during the exchanges into 10 mM sodium phosphate (pH 7.0) and 100 mM sodium acetate (pH 7.2).14 Following batch-processing with DEAE, the filtrate was concentrated, dialyzed against 100 mM sodium acetate (pH 7.2) and then purified via affinity chromatography on sepharose 6B.15 GAOX was eluted from the column in the same buffer. The fractions were subjected to denaturing gel electrophoresis (10% SDS-PAGE) and analyzed for concentration and specific activity using UV-vis spectroscopy. Fractions containing GAOX at a constant specific activity were pooled and dialyzed against 50 mM sodium phosphate (pH 7.0). Dialyzed protein was concentrated under pressure to 3 mg/mL, assayed for specific activity and O2 consumption, and then snap-frozen in N2(l) for storage at −80 °C. Prior to use, GAOX stored at −80 °C was thawed on ice, mixed by pipet and assayed for concentration, specific activity, and O2 consumption.
Concentration and Specific Activity
The concentration of GAOX was determined using published extinction coefficients at 280 nm of 1.05 × 105 M−1cm−1 and 1 OD = 0.65 mg/ml.16 Specific activity was measured at pH 7.0 and 25 °C using 60 mM 3-methoxybenzyl alcohol as the substrate. The extinction coefficient of the 3-methoxybenzaldehyde product is Δε = 2691 M−1cm−1,13 providing a rapid spectroscopic method to determine activity levels.
Activation
As isolated GAOX is a mixture of active enzyme possessing TyrCys• and inactive enzyme wherein the TyrCys radical has been reduced. In order to obtain a homogeneous sample of active radical-containing enzyme, GAOX was activated by brief incubation with 1:10 volume of enzyme: 50 mM K3Fe(CN)6 followed by purification on a MicroBioSpin P-6 column.17 Protein eluted from the column was mixed by pipet then assayed for concentration and specific activity.
Steady-state Measurements
Oxygen uptake experiments were conducted using a Clark type electrode (YSI). For measurements conducted at less than or equal to 350 μM O2, the electrode was calibrated using the concentration of dissolved oxygen in water under air-saturating conditions at 10 °C (1V = 352 μM). For measurements taken at greater than 350 μM O2, the electrode was calibrated with 100% O2 at 10 °C (1V = 1686 μM). Reaction volumes were 1 mL with final concentrations of 50 mM sodium phosphate (pH 7.0), 1-500 mM H2-OMeGal and 1 mM K3(FeCN)6. Ferricyanide was included in the reaction mixture to ensure full enzyme activity. Stock solutions of the sugar and ferricyanide were prepared in 50 mM sodium phosphate (pH 7.0) and adjusted for pH. After equilibration of the reaction mixture at 10 °C and the desired concentration of O2 (40-1000 μM), 1-4 μL of 2 μM activated enzyme was added via syringe. The reaction was monitored by following the change in concentration of dissolved O2 with respect to time. To minimize variability in O2 consumption rates between enzyme aliquots, a standard reaction under air-saturating conditions with 50 mM substrate and 4 nmol GAOX was measured daily and normalized to 225 s−1. All subsequent measurements collected on a given day were adjusted according to the ratio obtained from the standard reaction.
Kinetic Isotope Effects
Experiments with deuterated substrate, D2-OMeGal, were carried out at 10 °C in 50 mM sodium phosphate (pH 7.0) and 1 mM K2Fe(CN)6. The sugar concentration was maintained at 200 mM while O2 concentration was varied between 12 and 350 μM utilizing a mixture of N2(g) and air.
pH Dependence of Oxidative Half-Reaction
The effect of pH on kcat/Km(O2) was evaluated under steady-state conditions at 10 different pH values (pH 6-8, 50 mM sodium phosphate, pH 8-9, 20 mM sodium pyrophosphate, pH 9-10.5, 100 mM glycine/NaOH). The concentration of H2-OMeGal was held constant at 200 mM while [O2] was varied from 40-350 μM.
Solvent Isotope Effects
The solvent isotope effect on kcat/Km(O2) was measured under steady-state conditions in D2O at 7 different pD values (pD 6-8, 50 mM sodium phosphate, pD 8-9, 20 mM sodium pyrophosphate). The concentrations of H2-OMeGal and O2 were the same as in the experiments at varying pH values. Stock solutions were prepared by dissolving the buffer salts and H2-OMeGal in D2O and then lyophilizing. Following a second lyophilization the samples were re-dissolved in D2O to the desired concentration and adjusted to the appropriate pD using NaOD or D3PO4. The pD was determined by measuring the pH and adding 0.4 to the value.
18O Isotope Effects
18O kinetic isotope effects (KIEs) were measured competitively as described previously.18,19 Reactions with GAOX (0.5-8 nM) were carried out in 50 mM sodium phosphate (pH 7.0) at 10 °C with ∼1 mM O2 and 50 mM H2-OMeGal or D2-OMeGal. The reaction was coupled with horseradish peroxidase (200 nM) using guaiacol (3 mM) as the substrate to convert H2O2 to H2O. The 18O/16O ratios of residual oxygen were measured using isotopic ratio mass spectrometry (Department of Earth and Planetary Science, UC Berkeley, CA). The 18O KIEs are expressed as a ratio of ratios according to eq (1), where Rf is the 18O/16O isotopic ratio of substrate at f fractional conversion and R0 is the isotopic ratio of the blank.
| (3) |
Calculation of 18O Equilibrium Isotope Effects
The equilibrium isotope effects (EIEs) can be expressed as a product of three terms, contributed from the zero-point energies (ZPE), excited vibration states (EXC), and the mass and moments of inertia (MMI): EIE = ZPE × EXC × MMI.18 All three terms are related to vibrational frequencies of 18O– and 16O–containing reactants and products, as described previously.19 For the Cu(II)(OOH) species, Cu-O and O-O experimental frequencies were used to calculate the 18O EIEs given in Table 3, in a manner similar to previous reports.20 The populations of the two possible isotopic distributions (e.g. CuII–18O16OH or CuII–16O18OH) are expected to be close to each other, hence the 18O EIE was calculated using the formula: 18EIEcalc = 2/(18,16K−1 + 16,18K−1).19 For the Cu(II)(OOH) species, the frequencies and isotope shifts used for the O-H stretch and O-O-H bending modes were taken from Tian et al.19
Table 3.
Comparison of Measured and Calculated Kinetic and Equilibrium O-18 Isotope Effects
3. Results
Expression and Purification
Analysis of purified GAOX by denaturing gel electrophoresis (10 % SDS-PAGE) yielded a band accounting for greater than 95 % of the sample with an estimated molecular weight of 65 kD, consistent with the reported mobility for mature enzyme.21 All of the enzyme aliquots stored at −80 °C yielded the same concentration and specific activity after thawing. Following activation, the aliquots produced an average specific activity of 150 U/mg using the 3-methoxy benzyl alcohol assay, in accordance with reported literature values of 140 and 166 U/mg.22,23 The 3-methoxy benzyl alcohol assay provided a quick and easily comparable spectrophotometric method for assessing the condition and activity of GAOX before proceeding with kinetics measurements utilizing the more reactive 1-O-methyl-α-D-galactopyranoside as the substrate.
Steady-State Kinetics
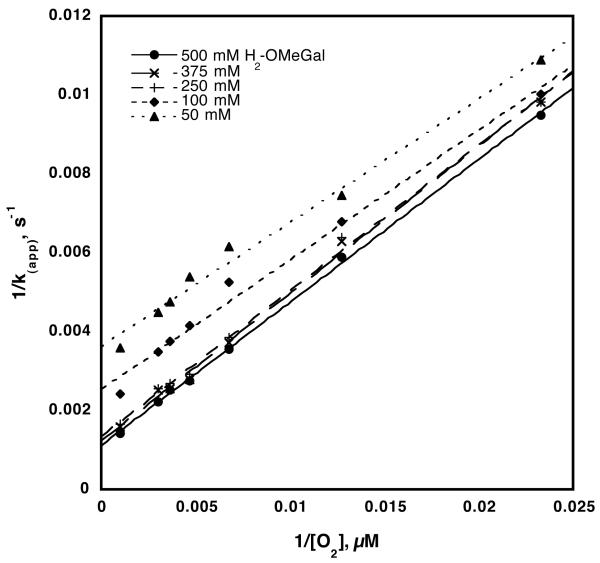
Steady-state kinetics information was obtained by measuring the rate of O2 consumption at a constant oxygen concentration while varying the H2-OMeGal concentration between 1 and 500 mM. Plots of the rate of O2 consumption (s−1) versus substrate concentration (mM) were fitted to the Michaelis-Menten equation and showed that both k(app) and Km for the sugar increased at higher oxygen concentrations, while the value of kcat/Km for sugar remained constant. The rate of O2 consumption vs. O2 concentration was analyzed as a function of varied substrate in a similar fashion, yielding values for kcat/Km(O2) that remained independent of sugar concentration. The latter feature is illustrated by a reciprocal plot of 1/k(app) vs. 1/[O2] at 5 different sugar concentrations yielding a series of parallel lines with slopes equal to Km/kcat(O2), Figure 1. The limiting value of kcat at saturating oxygen and substrate, as well as kcat/Km(OMeGal) at saturating oxygen and kcat/Km(O2) at saturating sugar are given in Table 1.
Figure 1.
Reciprocal plot of rate constants for GAOX vs. [O2], to illustrate the ping-pong nature of the GAOX reaction. All reactions were in 50 mM sodium phosphate (pH 7.0) and 1 mM K3Fe(CN)6 at 10 °C. The average calculated value for kcat/K(O2) was 2.6 ± 0.1 × 106 M−1s−1.
Table 1.
Kinetic Parameters for GAOX at 10 °C, pH 7.0 with Protio- and Deuterio- Substrates.
| Parameter | H2-OMeGal | D2-OMeGal |
|---|---|---|
| kcat, s−1 | 1165 (5) | 49 (2) |
| kcat/Km(OMeGal), M−1, s−1 | 8.3 (0.6) × 103 | 2.6 (0.4) × 102 |
| kcat/Km(O2), M−1, s−1 | 2.6 (0.3) × 106 | 1.5 (0.2) × 106 |
| Km(OMeGal), mM | 1.4 (0.1) × 102 | 1.9 (0.2) × 102 |
| Km(O2), μM | 4.4 (0.3) × 102 | 77 (10)a |
Value was calculated from kcat and kcat/Km(O2).
Deuterium Isotope Effects
Steady-state kinetic measurements were conducted with substrate that was dideuterated at position C-6 to assess the extent to which hydrogen atom transfer is involved in the rate-determining step of the oxidative half-reaction. Rate measurements vs. varying [O2] were carried out at a single concentration of D2-OMeGal (200 mM), giving an apparent kcat of 23.1 ± 0.4 s−1, a Km for O2 of 15 ± 2 μM and a kcat/Km(O2) of 1.5 ± 0.2 × 106 M−1s−1. Alternatively, keeping [O2] at 350 μM, rates were measured at variable [D2-OMeGal,] giving a kcat of 49 ± 2 s−1, a Km for D2-OMeGal of 1.90 ± 0.2 × 102, and a kcat/Km (D2-OMeGal) of 2.6 ± 0.4 × 102 M−1s−1. The resulting small substrate deuterium isotope effect on kcat/Km(O2) (to be discussed later), together with the large isotope effect on kcat, leads to a significantly reduced Km for O2 when using deuterated sugar. Thus, an experimental oxygen concentration of 350 μM is high enough to ensure O2 saturation leading to an excellent approximation of the true kcat and kcat/Km(D2-OMeGal) at saturating [O2], Table 1. By contrast, the analysis of rate vs. [O2] at about Km concentration for D2-OMeGal is expected to provide a plateau region approximately one-half kcat, compatible with the observed value of 23.1 s−1. Given the ping-pong nature of the reaction (cf. Figure 1) the values for kcat/Km(H2-OMeGal) and kcat/Km(O2) and their respective isotope effects will be independent of the concentration of the alternate substrate. The isotope effects resulting from deuterated substrates are in Table 2. We note that Whittaker et al.3 had earlier reported a limiting deuterium isotope effect of 8 ± 1 at saturating substrate and a single concentration of O2. While this was attributed to the O2 half reaction, the ca. 6-fold reduction in the Km(O2) value for deuterio- substrate (cf. Table 1) obviates any clear-cut mechanistic conclusion regarding the role of substrate-derived deuterium in the oxidative half reaction.
Table 2.
Kinetic Deuterium Isotope Effects for GAOX at 10 °C, pH 7.0.
| Parameter | Value |
|---|---|
| Dkcat, s−1 | 24 (1) |
| D(kcat/Km)(OMeGal) | 32 (5) |
| D(kcat/Km)(O2) | 1.6 (0.3) |
| D2O(kcat/Km)(O2)a | 1.0 (0.3) |
| D2O(kcat/Km)(O2)b | 1.9 (0.7) |
Measured at a fixed sugar and variable O2. H2-OMeGal in H2O vs. H2-OMeGal in D2O.
Double isotope effect. H2-OMeGal in H2O vs, D2-OMeGal in D2O.
The involvement of proton transfer in the rate-determining step of the oxidative half-reaction was assessed through steady-state measurements in D2O at a constant level of sugar, 200 mM H2-OMeGal. At a pD of 7.0, kcat/Km(O2) was 2.5 ± 0.7 × 106 M−1s−1 yielding a solvent isotope effect of 1.0. Experiments utilizing deuterated sugar in D2O resulted in kcat/Km(O2) = 1.3 ± 0.3 × 106 M−1s−1 and a “double” kinetic deuterium isotope effect of 1.9.
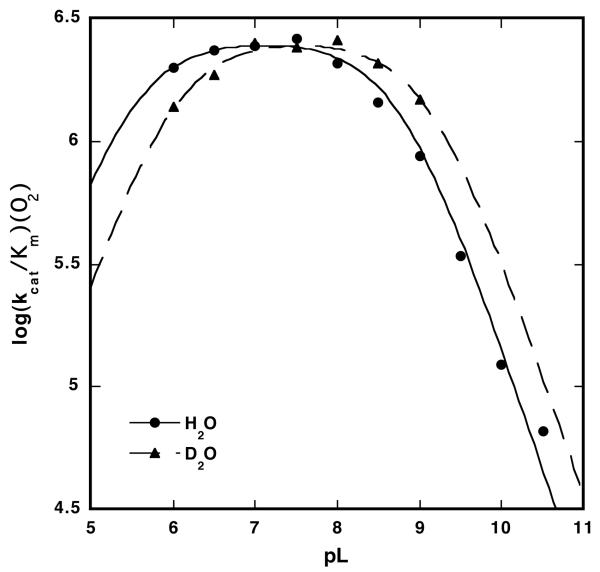
pH Effect
Measurements of kcat/Km(O2) at pH values between 6 and 10.5 were utilized to evaluate the involvement of ionizable groups in the rate-determining step of the oxidative half-reaction. Varying the pH in increments of 0.5 pH units revealed a maximum in kcat/Km(O2) between pH 6.5 and 7.5 (Figure 2). Increasing the pH from 7.5 to 10.5 resulted in a decrease of kcat/Km(O2) by approximately 2 orders of magnitude. The plot of log kcat/Km(O2) versus pH was fitted to an acidic and basic pKa (pKa1 = 5.4(0.3) and pKa2 = 8.76(0.04)). When the pD was varied between 6.0 and 9.0, a maximum was seen in log kcat/Km(O2) between pD 7.0 and 8.0. Below pD 7.0 and above pD 8.0, a decrease in log kcat/Km(O2) was observed (pKa1 = 6.1(0.1) and pKa2 = 9.14(0.05)). The overall profile obtained by varying pD is similar to that seen from varying the pH except that the curve is shifted to the right, along the x-axis.
Figure 2.
Plot of log(kcat/Km(O2)) vs. pL at 10 °C. Reactions contained 200 mM H2-OMeGal, 40-350 μM O2, and 2 nM GAOX in a solution of 50 mM sodium phosphate and 1 mM K3Fe(CN)6. Eq: log(kcat/Km) = log(kcat/Km)max – log(1+10pKa1 – pK + 10pL – pKa2).
18O Isotope Effects
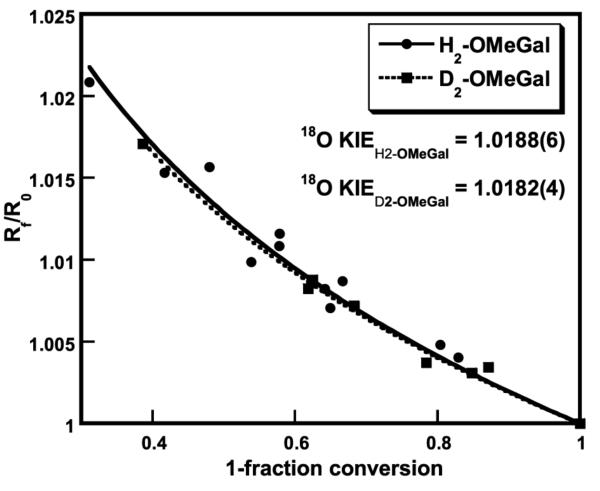
To obtain additional information on the interaction of reduced GAOX with O2 to produce hydrogen peroxide and oxidized GAOX, the 18O kinetic isotope effects (18O KIEs) were measured. Competitive 18O KIEs were determined from the fractionation of oxygen isotopes, i.e. the change in 18O/16O during the consumption of O2 catalyzed by GAOX. Isotope fractionation plots (18O/16O isotopic ratios of ratios versus fractional conversion) for the reduction of O2 by GAOX using H2-OMeGal and D2-OMeGal as substrates are shown in Figure 3. The data are well fitted by eq (1) to give 16O/18O KIE values of 1.0188 ± 0.0006 and 1.0182 ± 0.0004 for H2-OMeGal and D2-OMeGal, respectively. If, as an alternative to fitting the data via eq (3), the oxygen isotope effects are calculated at each fractional conversion, the 18O KIE is 1.0197 ± 0.002 for H2-OMeGal and 1.0191 ± 0.003 for D2-OMeGal. This approach leads to slightly larger values, with greater errors. In either case, the results show clearly that changes in oxygen bond order are occurring in the rate-determining step of kcat/Km(O2). The values for the protiated and deuterated substrates are identical within experimental error, indicating no substrate deuteration effect on the 18O KIE.
Figure 3.
Isotope fractionation plots for reaction of GAOX (10 °C, pH 7) with H2-OMeGal (●, fit in solid line) and D2-OMeGal (■, fit in dashed line). 18O KIE values were obtained by fitting to eq. (3).
4. Discussion
Demonstration of a Steady State Kinetic Ping-Pong Mechanism for Galactose Oxidase
In enzyme reactions involving multiple substrates, the kinetic mechanism can be elucidated through rate measurements in which the concentration of one substrate is varied while the alternate substrate concentrations are kept constant. This was accomplished for galactose oxidase by varying the concentration of O2 at different fixed levels of 1-O-methyl-α-D-galactopyranoside. As illustrated in Figure 1, reciprocal plots of k(app) vs. [O2] at varying concentrations of H2-OMeGal show close to parallel lines, consistent with a steady-state ping-pong mechanism wherein the reductive and oxidative reactions half-reactions (Scheme 1) are separated by an irreversible step. A ping-pong mechanism was predicted for GAOX on the basis of stopped flow experiments,3,4 but had not been previously shown under steady-state conditions. The values for kcat and Km(H2-OMeGal), Table 1, are in agreement with literature reports of 1180 s−1 and 175 mM for reaction of GAOX with galactose at 20 °C and pH 7.0.24 The value for kcat/Km(H2-OMeGal) compares favorably with reported second-order rate constants in the range of 1 × 104 M−1s−1.3,4 The much faster value of kcat/Km(O2), 2.6(0.3) × 106 m−1s−1, is also in the range of previously reported values for second-order rate constants of 8 × 106 M−1s−1 at 4 °C and 1 × 107 M−1s−1 at 25 °C.3,4 However, prior efforts to obtain Km(O2) for galactose oxidase were unsuccessful and it was concluded that the enzyme could not be saturated with oxygen.24,25 In contrast to earlier steady-state work, the current experiments were conducted at 10 °C slowing the reaction sufficiently for observation of saturation kinetics with [O2]; this results in the ability to detect the diagnostic pattern of non-intersecting lines characteristic of ping-pong kinetics, Figure 1. The experimental value for Km(O2) reported herein is of the same order of magnitude as the concentration of dissolved oxygen in water, a not surprising result given that galactose oxidase is an extracellularly secreted enzyme.
Deuterium Kinetic Isotope Effects Indicate Retention of Substrate-Derived Deuterium in the Reduced Form of GAOX
Kinetic isotope effects were utilized to elucidate the rate-determining steps of the two half-reactions. Large deuterium isotope effects of 24 ± 1 and 32 ± 5 were observed on kcat and kcat/Km(OMeGal) for the reductive reaction (10 °C), which can be compared to previously reported values of 21.2 and 22.5 obtained at 4 °C under conditions of low substrate concentration by stopped flow and steady-state methods, respectively. The large size of the KIE, together with the previously reported temperature dependence of this parameter, support a tunneling mechanism for H-atom abstraction from the sugar by the tyrosyl radical cofactor.3
Measurement of kcat/Km(O2) allows for the observation of isotope effects on the portion of a reaction that begins with the binding of oxygen through the first irreversible step of oxygen reactivity. The ability to separate the two half-reactions of GAOX, eqs. (1) and (2), would imply that deuteration of the sugar substrate will have no impact on the kcat/Km(O2) for enzyme recycling by oxygen. However, a small but real D(kcat/Km(O2) of 1.6 to 1.9, Table 2, indicates that the deuterium abstracted from the sugar remains in the active site. The fact that the solvent isotope effect on kcat/Km(O2) is unity, together with the fact that the observed value for D(kcat/Km(O2)) is similar in H2O and D2O, argues that bulk solvent is not able to penetrate the active site of the reduced enzyme. The carryover of the isotope from the reductive reaction to the oxidative reaction is likely a function of the active site tryptophan (W290), which is involved in π-stacking with the Tyr-Cys cofactor.26 Modification of this residue has been shown to dramatically slow the rate of catalysis while increasing solvent accessibility to the cofactor, as evidenced by rapid decay of the radical species.27 It is certainly expected that solvent will be accessible to the active site during certain stages of the catalytic cycle, e.g. in the Cu(II)-TyrCys• enzyme state that forms during H2O2 release and substrate binding. However, the present data appear to rule out solvent access during the oxidative half reaction that involves Cu(I)-TyrCys.
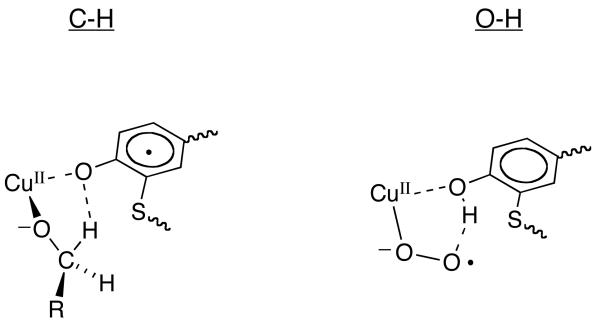
By analogy to the steps proposed for the reductive half-reaction, the rate-determining step of the oxidative half-reaction is hypothesized to be an H-atom transfer from the cofactor to metal-bound superoxide. Typical KIEs for H-atom transfer reactions involving tunneling are in the range of 10-100.28,29 One possibility for the small deuterium KIE observed herein for kcat/Km(O2) is that the hydrogen atom transfer in the oxidative half-reaction of GAOX is occurring between two heteroatoms, O–H---O. In general, smaller primary deuterium isotope effects are anticipated for such reactions,28 in comparison to hydrogen atom abstraction from carbon.29 However, a value of 1.6 to 1.9 would appear outside the range anticipated for a rate-limiting O–H abstraction via tunneling, raising the possibility that the O-H bond cleavage is only partially rate-determining for the measured kcat/Km(O2).
Impact of Substrate Deuteration on Oxygen-18 Kinetic Isotope Effects Implicates a Single Rate-Limiting H-Transfer Step for Oxidation of Reduced Cofactor by O2
The use of O-18 kinetic isotope effects to establish rate-determining steps for reactions involving O2 has seen increasing application in the recent literature. Competitive 18O kinetic isotope effects (18kcat/Km(O2)) were carried out for GAOX to assess the extent to which a change in oxygen bond order occurs in all reversible steps involving O2 binding through the first irreversible step. The experimental 18O KIE values for GAOX are 1.0188 ± 0.0006 and 1.0182 ± 0.0004 using H2-OMeGal and D2-OMeGal, respectively, Table 2. These results both identify electron transfer to O2 as a rate-limiting step and eliminate a partially rate-determining binding of O2 to GAOX. If the latter had been the case, the 18O KIE would be predicted to increase upon substrate deuteration, as the contribution of hydrogen transfer to kcat/Km(O2) is enhanced relative to reaction with protio-substrate. The combined observations of the measured 18O KIE being independent of substrate deuteration, while substrate deuteration reduces the magnitude of kcat/Km(O2), implicates a single rate-determining step for hydrogen abstraction from reduced cofactor in the oxidative half-reaction. In this manner the observed small impact of substrate deuteration on kcat/Km(O2) is concluded to be an intrinsic property of the oxidative half-reaction, in marked contrast to the enormous non-classical KIE observed for the reductive half-reaction.
One feature that will affect the size of the measured isotope effect is the reaction driving force and whether hydrogen transfer takes place from the ground state vibrational level of reactant to the ground state vibrational level of product (0 → 0 transition). In the case of quite a few enzymatic reactions that catalyze hydrogen atom abstraction from carbon, the elevated size of the experimental substrate deuterium isotope effect and its weak temperature dependence can be explained via an electronically and vibronically non-adiabatic treatment in which the majority of hydrogen transfer occurs from the lowest vibrational level of the reactant. A contrasting trend has been seen in numerous hydride transfer reactions where the primary deuterium isotope effect is small and in the range of kH/kD = 3 to 4, although considerable evidence has been amassed for hydrogen tunneling in these systems as well.30 Hammes-Schiffer and her co-workers have been able to rationalize the differences between the large isotope effects in the free radical based hydrogen atom transfers in enzymes vs. hydride transfer reactions via the use of an electronically adiabatic treatment in the latter case.31 That is, theoretical treatment of systems in which there is substantial electronic interaction between the hydrogen donor and acceptor reproduces the small experimental isotope effects. It, thus, seems very likely that the most fundamental difference between the C-H and O-H activation steps catalyzed by GAOX lies in the degree of ground state interaction between the hydrogen donor and acceptor, with this being much greater for the half-reaction in which a hydrogen atom is removed from oxygen. The greater electronic interaction in the O-H---O transfer is also expected to decrease the heavy atom donor to heavy atom acceptor distance, which will further decrease the size of the isotope effect. Though a formal theoretical treatment of GAOX is outside the scope of this study, the present results offer the opportunity to explore how a single enzyme active site controls hydrogenic wave function overlap for an adiabatic (O-H---O) vs. non-adiabatic (C-H---O) process.
The Magnitude of the 18O KIE in GAOX Provides a Frame of Reference for Oxygen Activation at a Cu+1 Center
A very surprising feature from this study is an O-18 kinetic isotope effect for GAOX that is remarkably close to values measured previously for DβM and PHM, Table 2, providing corroborative evidence for a reactive copper-superoxo species as the oxygenating agent in the latter enzyme systems. Inspection of kcat/Km(O2) values, under conditions where this parameter is limited by the hydrogen transfer step, indicates a ten-fold or larger rate constant for GAOX than for the copper monooxygenase family: 2.5 × 106 M−1s−1 for GAOX vs. 0.05 to 2.6 × 105 M−1s−1 for PHM and DβM, depending on the substrate oxidized. The faster rate for the GAOX oxidative half-reaction is in accord with model studies that have demonstrated a lower barrier for hydrogen abstraction from oxygen than carbon.32 In addition to differences in the magnitude of kcat/Km(O2), X-ray structures for the active sites of GAOX and PHM indicate very different copper geometries, active site structures and access to bulk solvent. The fact that such different enzyme systems display almost identical values for the 18O KIE establishes a value of ≥1.019 as a frame of reference for abstraction of a hydrogen atom at a copper superoxo- center.
As argued in previous publications, reaction of oxygen at a metal center in the absence of O-O bond cleavage may be expected to produce a kinetic isotope effect that will be fairly insensitive to the reaction coordinate frequency.33 Under this condition, the equilibrium O-18 isotope effect can provide an estimate for the upper limit of the measured kinetic isotope effect, calculated in Table 3 for formation of Cu(II)-OOH from Cu(I) and O2 using experimental frequencies and isotope shifts for the indicated complexes (models I and II) or DFT calculations (model III). Roth has advocated the use of DFT calculations to obtain a full-range of vibrational frequencies when estimating equilibrium O-18 isotope effects, noting that in some instances the use of the more limited experimental frequencies can lead to an over-estimate of the values for the isotope effect.34 We consider the O-18 equilibrium isotope effects for Cu(II)(-OOH) formation within models I and II, Table 3, a reasonable starting frame of reference for the present studies, while the value for model III, Table 3, appears much too low to be relevant to the kinetic isotope effects for GAOX and PHM/DβM.
The present results are relevant to a recent study of the reaction mechanism for the oxidative half-reaction of the copper-dependent pea seedling oxidase (PSAO)35. The size of the 18O KIE played a dominant experimental role in the conclusion of a rate-limiting outer sphere electron transfer from the reduced active site cofactor to a pre-formed Cu(II)(O2·−)36. The authors report an O-18 KIE of 1.0136, considerable smaller than the KIE data now available for GAOX and for PHM/DβM. It could be argued that the mechanism put forth for the PSAO reaction is sufficiently different from the outer sphere hydrogen atom transfer to the pre-formed Cu(II)(O2·−) in GAOX and PHM/ DβM, such that the O-18 may be expected to be of a different magnitude. However, as has been shown quite convincingly in previous analyses of the reductive activation of O2, formation of a hydroperoxo anion predicts a much larger KIE than for production of hydrogen peroxide, Table 3, a result of decreased bonding to the reactive oxygen in the hydroperoxo- anion case. Thus, based on the present studies, the mechanism proposed for PSAO in ref (35) would predict an 18O KIE at least as large as 1.018. It remains possible that an inner sphere oxidative half-reaction is operating in PSAO: however, the conclusion of such a mechanism based on the magnitude of the experimentally available 18O KIE requires further examination.
Scheme 2.
Comparison of C-H vs. O-H Activation Steps in GAOX.
Acknowledgment
This work was supported by grants from the National Institutes of Health (GM025765 to J.P.K. and F32 GM065010 to K.J.H.).
Abbreviations
- GAOX
galactose oxidase
- DβM
dopamine β-monooxygenase
- PHM
peptidylglycine α-hydroxylating monooxygenase
- TβM
tyramine β-monooxygenase
- DFT
density functional theory
- H2-OMeGal
1-O-methyl-β-D-galactopyranoside
- D2-OMeGal
1-O-methyl-[6,6-2H2]-α-D-galactopyranoside
- KIE
kinetic isotope effect
- EIE
equilibrium isotope effect
References
- 1.Rogers MS, Baron AJ, McPherson MJ, Knowles PF, Dooley DM. J. Am. Chem. Soc. 2000;122:990–991. [Google Scholar]
- 2.Whittaker MM, Whittaker JW. J. Biol. Chem. 2003;278:22090–22101. doi: 10.1074/jbc.M300112200. [DOI] [PubMed] [Google Scholar]
- 3.Whittaker MM, Ballou DP, Whittaker JW. Biochemistry. 1998;37:8426–8436. doi: 10.1021/bi980328t. [DOI] [PubMed] [Google Scholar]
- 4.Borman CD, Saysell CG, Sykes AG. J. Biol. Inorg. Chem. 1997;2:480–487. doi: 10.1021/ic970255m. [DOI] [PubMed] [Google Scholar]
- 5.Klinman JP. Chem. Rev. 1996;96:2541–2561. doi: 10.1021/cr950047g. [DOI] [PubMed] [Google Scholar]
- 6.Klinman JP. J. Biol. Chem. 2006;281:3013–3016. doi: 10.1074/jbc.R500011200. [DOI] [PubMed] [Google Scholar]
- 7.Chen P, Solomon EI. Proc. Natl. Acad. Sci. 2004;36:13105–13110. doi: 10.1073/pnas.0402114101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crespo A, Marti MA, Roitberg AE, Amzel LM, Estrin DA. J. Am. Chem. Soc. 2006;128:12817–12828. doi: 10.1021/ja062876x. [DOI] [PubMed] [Google Scholar]
- 9.Evans JP, Ahn K, Klinman JP. J. Biol. Chem. 2003;50:49691–49698. doi: 10.1074/jbc.M300797200. [DOI] [PubMed] [Google Scholar]
- 10.Francisco WA, Merkler DJ, Blackburn NJ, Klinman JP. Biochemistry. 1998;37:8244–8252. doi: 10.1021/bi973004y. [DOI] [PubMed] [Google Scholar]
- 11.Chen P, Solomon EI. J. Am. Chem. Soc. 2004;126:4991–5000. doi: 10.1021/ja031564g. [DOI] [PubMed] [Google Scholar]
- 12.Prigge ST, Eipper BA, Mains RE, Amzel LM. Science. 2004;304:864–867. doi: 10.1126/science.1094583. [DOI] [PubMed] [Google Scholar]
- 13.Tressel P, Kosman DJ. Methods in Enzymol. 1982;89:163–171. doi: 10.1016/s0076-6879(82)89029-0. [DOI] [PubMed] [Google Scholar]
- 14.Amaral D, Bernstein L, Morse D, Horecker BL. J. Biol. Chem. 1963;238:2281–2284. [PubMed] [Google Scholar]
- 15.Hatton MWC, Regoeczi E. Methods in Enzymol. 1982;89:172–176. [Google Scholar]
- 16.Kosman DJ, Ettinger MJ, Weinger RE, Massaro EJ. Arch. Biochem. Biophys. 1974;165:456–467. doi: 10.1016/0003-9861(74)90271-9. [DOI] [PubMed] [Google Scholar]
- 17.Whittaker MM, Whittaker JW. Protein Expr. Purif. 2000;20:105–111. doi: 10.1006/prep.2000.1287. [DOI] [PubMed] [Google Scholar]
- 18.Bigeleisen J, Mayer MG. J. Chem. Phys. 1947;15:261–267. [Google Scholar]
- 19.Tian GC, Klinman JP. J. Am. Chem. Soc. 1993;115:8891–8897. [Google Scholar]
- 20.Roth JP, Klinman JP. In: Isotope Effects in Chemistry and Biology. Kohen A, Limbach H-H, editors. Taylor and Francis Inc.; Boca Raton, FL: 2006. pp. 645–669. [Google Scholar]
- 21.Baron AJ, Stevens C, Wilmot C, Seneviratne KD, Blakeley V, Dooley DM, Phillips SEV, Knowles PF, McPherson MJ. J. Biol. Chem. 1994;269:25095–25105. [PubMed] [Google Scholar]
- 22.Whittaker MM, Whittaker JW. J. Biol. Chem. 1988;263:6074–6080. [PubMed] [Google Scholar]
- 23.Tressel P, Kosman DJ. Anal. Biochem. 1980;105:150–153. doi: 10.1016/0003-2697(80)90437-6. [DOI] [PubMed] [Google Scholar]
- 24.Kwiatkowski LD, Adelman M, Pennelly R, Kosman DJ. J. Inorg. Biochem. 1981;14:209–222. doi: 10.1016/s0162-0134(00)80001-x. [DOI] [PubMed] [Google Scholar]
- 25.Villafranca JJ, Freeman JC, Kotchevar A. In: Bioinorganic Chemistry of Copper. Karlin KD, Tyeklar Z, editors. Chapman and Hall; New York: 1993. pp. 439–446. [Google Scholar]
- 26.Ito N, Phillips SEV, Stevens C, Ogel ZB, McPherson MJ, Keen JN, Yadav KDS, Knowles PF. Nature. 1991;350:87–90. doi: 10.1038/350087a0. [DOI] [PubMed] [Google Scholar]
- 27.Rogers MS, Tyler EM, Akyumani N, Kurtis CR, Spooner RK, Deacon SE, Tamber S, Firbank SJ, Mahmoud K, Knowles PF, Phillips SEV, McPherson MJ, Dooley DM. Biochemistry. 2007;46:4606–4618. doi: 10.1021/bi062139d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bell RP. The Proton in Chemistry. Cornell University Press; New York: 1973. pp. 250–296. [Google Scholar]
- 29.Knapp MJ, Rickert K, Klinman JP. J. Am. Chem. Soc. 2002;124:3865–3874. doi: 10.1021/ja012205t. [DOI] [PubMed] [Google Scholar]
- 30.Nagel ZD, Klinman JP. Chem. Rev. 2006;106:3095–3118. doi: 10.1021/cr050301x. [DOI] [PubMed] [Google Scholar]
- 31.Hammes-Schiffer S. Acc. Chem. Res. 2006;39:93–100. doi: 10.1021/ar040199a. [DOI] [PubMed] [Google Scholar]
- 32.Mayer JM. Annu. Rev. Phy. Chem. 2004;55:363–390. doi: 10.1146/annurev.physchem.55.091602.094446. [DOI] [PubMed] [Google Scholar]
- 33.Lanci MP, Brinkley DW, Stone KL, Smirnov VV, Roth JP. Angew. Chem., Int. Ed. 2005;44:7273–7276. doi: 10.1002/anie.200502096. [DOI] [PubMed] [Google Scholar]
- 34.Roth JP. Curr. Opin. Chem. Biol. 2007;11:142–150. doi: 10.1016/j.cbpa.2007.01.683. [DOI] [PubMed] [Google Scholar]
- 35.Mukherjee A, Smirnov VV, Lanci MP, Brown DE, Shepard EM, Dooley DM, Roth JP. J. Am. Chem. Soc. 2008;130:9459–9473. doi: 10.1021/ja801378f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.A second major argument for an inner sphere mechanism in PSAO came from a comparison of the experimental thermal activation parameters to reaction driving force using a thermodynamics cycle based on solution bond dissociation energies and pKa values. However, as the authors comment, the difference between the observed ΔG‡ = 5.6 ± 1.3 kcal/mol and the calculated ΔG‡ = 8.5 kcal/mol for reaction of the monoanion of the reduced cofactor could be accounted for by a strategically placed counterion or protein-derived electrostatics (Ref (35)).
- 37.Tian GC, Berry JA, Klinman JP. Biochemistry. 1994;33:226–234. doi: 10.1021/bi00167a030. [DOI] [PubMed] [Google Scholar]
- 38.Francisco WA, Blackburn NJ, Klinman JP. Biochemistry. 2003;42:1813–1819. doi: 10.1021/bi020592t. [DOI] [PubMed] [Google Scholar]
- 39.Yamaguchi S, Kumagai A, Nagatomo S, Kitagawa T, Funahashi Y, Ozawa T, Jitsukawa K, Masuda H. Bull. Chem. Soc. Jpn. 2005;78:116–124. [Google Scholar]