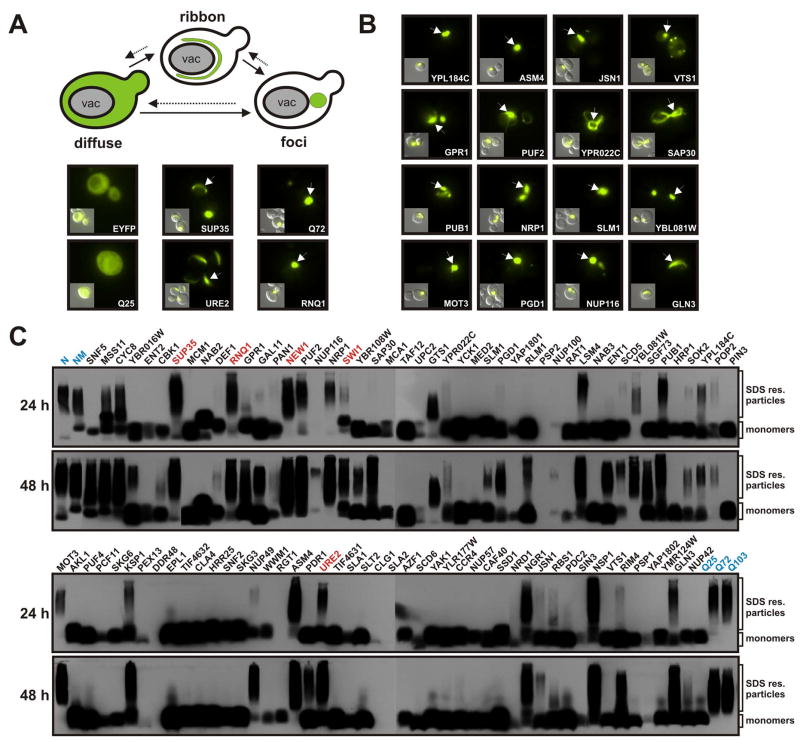
Figure 2. Prion domains form intracellular aggregates detectable by microscopy and SDD-AGE.
(A) Expression of amyloidogenic proteins in the yeast cytosol leads to the formation of ribbon and dot-like structures. cPrD-EYFP fusion proteins were expressed from a galactose-regulatable plasmid in yeast cells containing the [RNQ+] prion. The yeast cells were subjected to fluorescence microscopy after 24 h of expression. Representative fluorescence microscopy images are shown together with DIC images (insets). Arrows point to aggregates in the yeast cytosol.
(B) A selected set of cPrD candidates forming fluorescent foci after 24 h of expression. Conditions were as described in (A).
(C) Detection of SDS-resistant aggregates by SDD-AGE in cell lysates of yeast strains expressing cPrD-EYFP fusions. Expression of the proteins was induced for 24 h (top gels) or 48 h (bottom gels). Control proteins (highlighted in blue) were the N or NM domains of Sup35p (top left) and the huntingtin protein length variants Q25, Q72 and Q103 (bottom right). Proteins were detected with a GFP-specific antibody. Previously identified prions and the prion candidate New1p are highlighted in red.