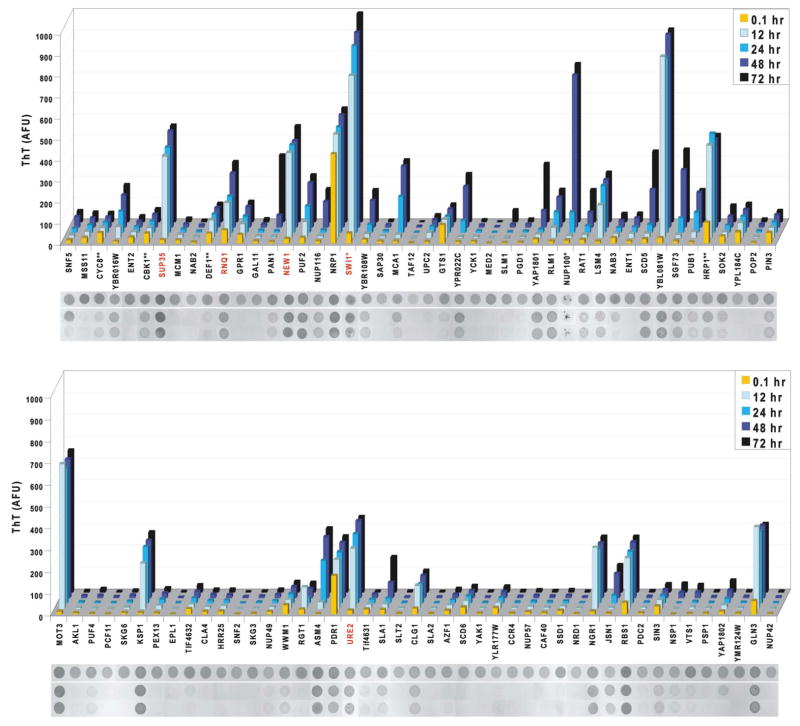
Figure 3. Prion domains have diverse amyloid propensities.
Amyloid formation of cPrD-M-His proteins was followed by ThT fluorescence (arbitrary units) measured at the indicated time points. Shown are means of three replicate assemblies (coefficients of variation were generally < 30; exceptions were Pan1, Nup116, and Yap1802, which each had highly variable lag phases). After the final measurement, reactions were analyzed for detergent-resistant aggregation. Aliquots from one experiment were spotted directly onto nitrocellulose (“total”), or treated with either 0.1% Tween 20 or 2% SDS and filtered through a non-binding membrane. Retained protein was visualized with Ponceau S. *These cPrDs were purified with a polyHis-tag only (no M). **These cPrDs were purified with the M-His tag at their N-terminus. Red labels indicate known prion proteins.