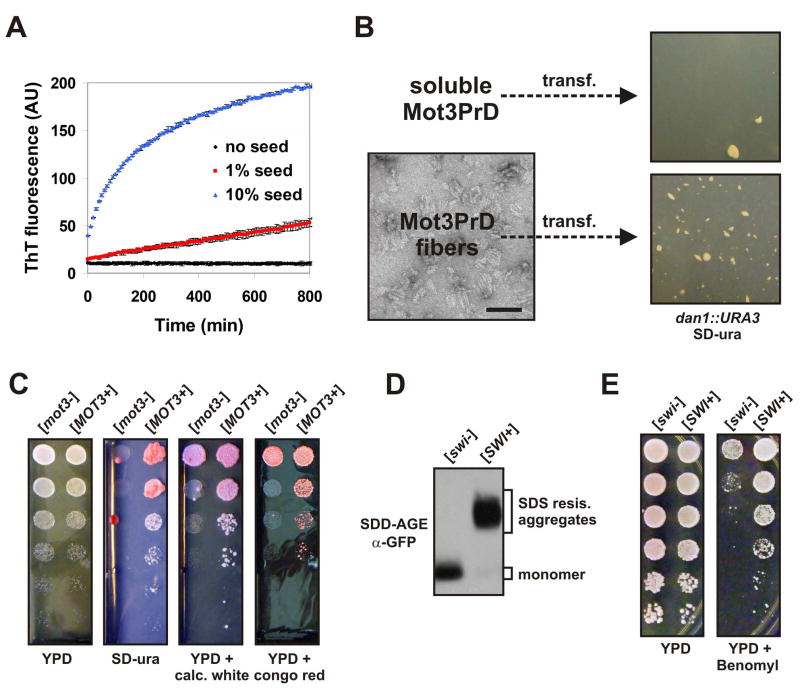
Figure 7. Mot3p and Swi1p form amyloid-based prions that can be beneficial.
(A) Mot3PrD amyloid fibers used for fiber transformation experiments were added at 1% or 10% (w/w) to fresh 20 μM Mot3PrD amyloid assembly experiments and monitored for acquisition of ThT fluorescence. Shown are means +/− STDV.
(B) Mot3PrD-M-His protein was polymerized and examined for a fibrillar amyloid morphology by transmission electron microscopy (bar = 100 nm). [mot3−] spheroplasts were transformed with either soluble (freshly diluted) or amyloid Mot3PrD-M-His protein and plated directly onto SD-ura plates containing 1 M sorbitol.
(C) [MOT3+] and [mot3−] isolates were grown over night in YPD, then spotted (serial 5 fold dilutions) to SD-ura, YPD, or YPD containing calcofluor white (50 μg/ml) or congo red (500 μg/ml).
(D) Lysates of diploid [swi−] and [SWI+] cells carrying an integration of the coding sequence for EGFP at one of the two chromosomal loci of the SWI1 gene were investigated by SDD-AGE and Western blotting. Swi1p-EGFP fusion proteins were detected using an anti-GFP antibody.
(E) 5-fold serial dilutions of [swi−] and [SWI+] cells were spotted on YPD and YPD containing benomyl (5 mg/l).