Fig. 8. Hypothesis for a novel physiological mechanism of up-regulation of functional α4β2.
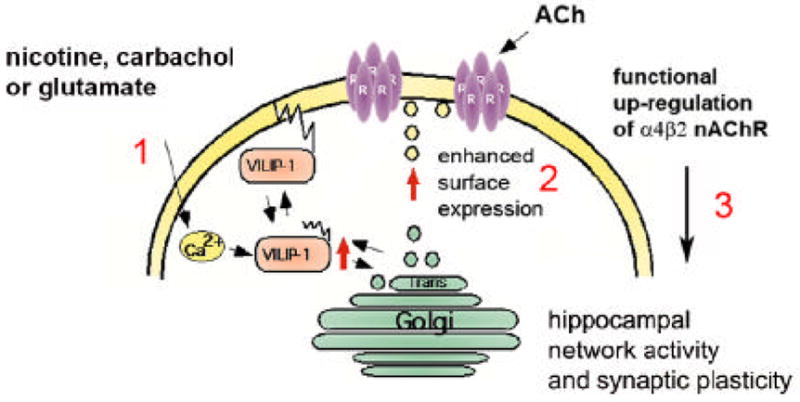
The cartoon shows the neuronal Ca2+-sensor VILIP-1, is activated following stimulation of a neuron by a signal, such as glutamate, carbachol or nicotine, which is acting on receptors increasing the intracellular Ca2+ level (1). VILIP-1 shuttles to cell surface and Golgi membranes as well as clathrin-coated vesicles, where it co-localizes with α4β2 nAChR and with syntaxin-6, a SNARE implicated in clathrin-dependent transport mechanisms in the trans-Golgi network. VILIP-1 causes enhanced exocytosis and surface transport of α4β2 nAChR (2), consequently increasing the surface expression and finally the sensitivity of the neuron towards ACh (3). The nicotine or glutamate induced translocation of VILIP-1 to cellular membranes (1, Zhao et al., 2008), and in turn the up-regulation of functional α4β2 nAChRs (2, Lin et al., 2002, this study) may contribute to plasticity of nicotinergic neurotransmission in principal neurons and/or in interneurons in the hippocampus, since the expression of VILIP-1 in conjunction with the α4β2 nAChR enhances the frequency of inhibitory postsynaptic currents (IPSCs) in hippocampal cultures (3, Gierke et al., 2008). Thereby, the physiological up-regulation of α4β2 nAChR by VILIP-1 might modulate hippocampal network activity and synaptic plasticity.
