Abstract
Osteoporosis is a skeletal metabolic disease characterized by a compromised bone fragility, leading to an increased risk of developing spontaneous and traumatic fractures. Osteoporosis is considered a multifactorial disease and fractures are the results of several different risk factors both extra- and intraskeletal. Thus bone fragility can be the end point of several different causes: a) failure to reach an optimal peak bone mass during growth; b) excessive bone resorption resulting in decreased bone mass and microarchitectural deterioration; c) inadequate formation upon an increased resorption during the process of bone remodeling. The pharmacological therapeutical options, available to date, are directed on prevention of fractures. The aim of this paper is to describe the activities and the mechanisms of action, as known at present, of the most used therapies for osteoporosis and their clinical implications. Improvement of knowledge in this field will allow us to further improve therapeutical choices and pharmacological interventions.
Keywords: Osteoporosis, estrogens, bisphosphonates, SERMS, teriparatide, mechanism of action, fracture
Introduction
Osteoporosis is a bone metabolic disease characterized by a compromised skeletal fragility, leading to an increased risk of developing spontaneous and traumatic fractures (NIH 2001). Osteoporosis has been defined a social disease due to its high impact on mortality and morbidity and to the alterations on the quality of life of patients affected (Kado et al 1999; Gold 2001).
Skeletal fragility can be the result of several different causes: a) failure to reach an optimal peak bone mass both in terms of mass and strength during growth; b) excessive bone resorption resulting in decreased bone mass and microarchitectural deterioration; c) inadequate formation upon an increased resorption during the process of bone remodeling. What is clear today is that this disease is mainly the consequence of an imbalance of the physiological process of bone turnover (or coupling), with the lack of the equilibrium between the activity of osteoblasts and osteoclasts.
The pharmacological therapeutical options, available to date, are directed on prevention of fractures and must be then chosen on their antifracturative efficacy, since fractures trigger back pain (vertebral fracture), limit activity, and often confine patients to bed (Klotzbuecher et al 2000; Lindsay et al 2001).
Additionally, multiple vertebral fractures cause kyphosis and loss of height and fracture at any site increases the risk for subsequent fracture: indeed up to 20% of women who have an incident vertebral fracture will develop a subsequent fracture within one year. Nowdays different therapeutical options can be classified according to the mechanism(s) of action by which prevent bone loss. Bisphosphonates (Diez-Perez 2002) and selective estrogen receptor modulators (SERMs) can be classified as antiresorptive agents since their main role is to block osteoclast activity and their effects are aimed to block either further decrease of bone density or deterioration of skeletal microarchitecture (Ettinger et al 1999; Chesnut et al 2000); in contrast, teriparatide (recombinant human parathyroid hormone), the newest agent against osteoporosis (Hodsman et al 2005), is anabolic with respect to bone and therefore, bone microarchitecture is restored with increase in both cortical thickness and connectivity.
The aim of this paper is to describe the currently known activities and the mechanisms of action of the most used therapies for osteoporosis and their clinical implications.
Physiopathological mechanism of bone homeostasis
Bone is a highly specialized form of connective tissue, whose primary functions are mechanical support, physical protection for organs and soft tissue, and storage for systemic mineral homeostasis. Indeed, the skeleton is an extremely complex tissue which, to accomplish the above mentioned functions, has and maintains contradictory properties: strength and lightness, stiffness and flexibility (Seeman 2003b). In this complex living tissue the extracellular matrix is mineralized, conferring marked rigidity and strength, but also maintaining some elasticity to allow flexibility (Seeman 2003b; Migliaccio et al 2004).
This sophisticated equilibrium is due to and maintained by a dynamic process, called remodeling, characterized by a balance, referred to as coupling, between the activity of osteoclasts, the bone resorbing cells and osteoblasts, the bone forming cells (Boivin and Meunier 2003; Seeman 2003b). During development and growth, bone formation exceeds bone resorption (modeling) with a net gain in bone mass (Raisz 2001; Boivin and Meunier 2003; Seeman 2003b) while in mature individuals, bone loss consequent to osteoclast resorption is replaced by appropriate bone formation, allowing bone restoration. Many factors, such as genetic, nutritional, hormonal, and environmental influence osteoclast and osteoblast differentiation, recruitment and activity, acting through life to maintain a physiological remodeling (Manolagas 2000; Migliaccio et al 2004). Recently attention has been focused on the role of local factors such growth factors and cytokines in the development of osteoporosis (Pacifici et al 1991; Jilka et al 1992; Raisz 1993). For instance, estrogens’ effects on osteoclast activity has been claimed to be both direct and indirect, through a paracrine regulation via osteoblast cytokines (Pacifici et al 1991; Jilka et al 1992; Raisz 1993; Hughes et al 1996; Yasuda et al 1998; Burgess et al 1999; Taranta et al 2002; Cenci et al 2003). Estradiol appears to decrease the responsiveness of osteoclasts to factors such as receptor activator of nuclear factor kappa B ligand (RANKL) and macrophage-colony stimulating factor (M-CSF), released in the bone microenvironment by osteogenic cells and by marrow stroma (Hughes et al 1996; Taranta et al 2002). Additionally, estrogen deficiency might also increase the lifespan of osteoclasts while the lifespan of osteoblasts would be decreased (Hughes et al 1996; Taranta et al 2002). Thus, bone loss accelerates in women after surgical or natural menopause because estrogen withdrawal is associated with increased remodeling intensity (activation frequency) in favor of bone resorption, with development of osteopenia.
Therefore, optimal antifracture efficacy results if drug therapy is targeted to the underlying cellular abnormality (anabolic therapy for osteoporotic individuals with reduced bone formation, antiresorptive therapy for patients with increased resorption).
Antiresorptive therapy
Antiresorptive agents decrease the number, activity, and life span of osteoclasts by restoring bone density by decreasing remodeling of bone. These agents are capable of preserving bone mass, enhancing mineralization of the bone matrix, potentially stabilizing the trabecular microarchitecture, and reducing fracture rates. However it must be pointed out that not all antiresorptives act in the same manner. For instance, Raloxifene (RAL), showed its efficacy by significantly increasing bone mineral density (BMD), with sustained effect during time, both at lumbar spine and at the hip (Bjarnason et al 2001; Delmas et al 2002). However, when subjects are categorized in tertiles of BMD change, there is no correlation between risk reduction of vertebral fractures and the tertiles of BMD change (Bjarnason et al 2001; Delmas et al 2002), strongly suggesting the importance and the role, beside the increase in bone density, of others issues not considered before in the fracture prevention risk.
Thus, this section will review the differential mechanism(s) of action of the most used antiresorptive molecules and their clinical use.
Mechanism of action of bisphosphonates
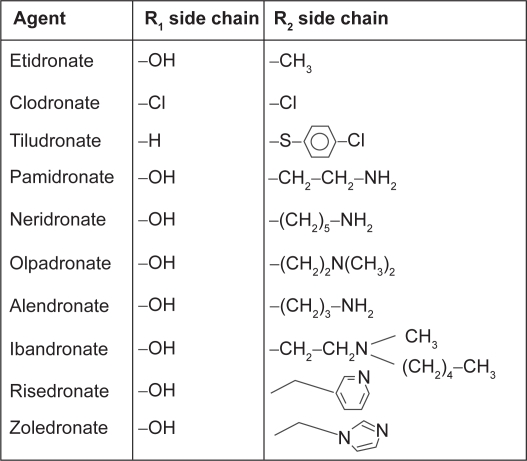
Bisphosphonates (BPs) are potent selective inhibitors of osteoclastic bone resorption and are commonly used clinically for the treatment and prevention of postmenopausal osteoporosis. These compounds are analogues of naturally occurring pyrophosphate (P-C-P) which bind to hydroxyapatite crystals. BPs are traditionally divided into non-nitrogen-containing and nitrogen-containing molecules. Clodronate, etidronate, and pamidronate belong to the first group while alendronate, risedronate, ibandronate, and zoledronate belong to the second group. The addition of nitrogen group in the molecules modifies their potency with a ratio ranging from 100:1 to 10000:1 (Figure 1). Nitrogen-containing BPs (N-BPs) reduce osteoclast function by inhibiting farnesyl diphosphate synthase, an enzyme which is active in the mevalonate pathway of the cholesterol biosynthesis. Depletion of farnesyl diphosphate or geranylgeranyl diphosphate levels limits the prenylation of small GTP-containing proteins (eg, Rho, Rac, cdc42, and Rab), essential for osteoclast functions and survival, leading to cell apoptosis (Reszka and Rodan 2004). At cellular level N-BPs blunt differentiation and recruitment of osteoclast precursors from the common hematopoietic stem cell, strongly decreasing the number of mature active osteoclasts. Additionally, N-BPs inhibit osteoclast adhesion to the mineralized matrix, reduce the osteoclast life span by activation of pro-apoptotic caspases, and directly inhibit osteoclast activity by alteration in the cytoskeleton, including cell morphology, integrin signaling, and disruption of the ruffled border and trafficking of the endosomes (Benford et al 2001).
Figure 1.
Chemical structures of bisphosphonates.
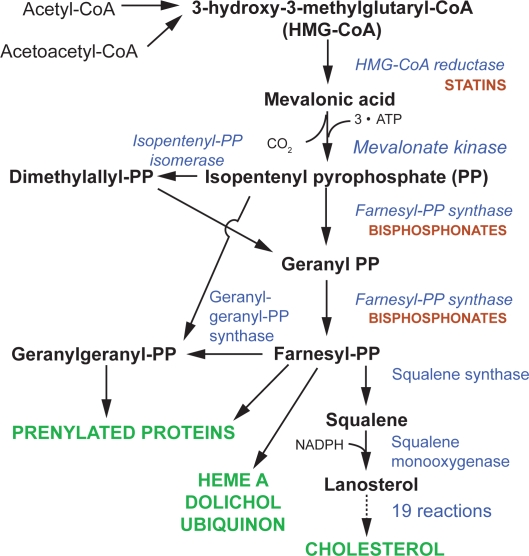
In contrast, non-N-BPs have little effect on the mevalonate pathway, but instead they are incorporated into intracellular adenosine triphosphate (ATP) analogues that have no releasable energy content, thus leading to cell death (Figure 2). Subsequent to this str ong inhibition of osteoclast activity, bone resorption is almost completely blocked at the level of basic multicellular units, BMU (Seeman 2003a), with a consequent blockade of bone remodeling.
Figure 2.
Schematic representation of the mevalonate pathway and the effects of the nitrogen-containing bisphosphonate.
Thus, BPs combat the three mechanisms causing bone loss by: a) reducing the rate of bone remodeling; b) reducing the negative balance in the bone modeling BMU by decreasing number and depth of resorption cavities; and c) increasing the mineral content (density) of the bone (Seeman 2003a). However, studies carried on in experimental animal models would indicate that BPs in part differ in their mode of action suggesting, for instance, that risedronate reduces resorption depth and increases mean wall thickness while alendronate reduces cortical porosity (Boyce et al 1995; Roschger et al 2001).
Bisphosphonates are retained over time in the skeleton and may exert long-term effects and their biological half-lives may vary and depend on the rate of bone turnover, potency, and binding affinity of each one to bone. An extended half-life may be beneficial, because it may prolong a residual effect of the agent after treatment discontinuation. On the other hand, prolonged marked suppression of bone turnover may have a theoretical negative effect on the ability of bone cells to repair microdamages with consequent deterioration of bone quality.
Interestingly, the different potency of the BPs allows differential dosages and pattern of administration (orally vs intravenously, daily vs weekly, or bi/monthly), which allows a better personalization of therapeutical intervention.
Clinical use of bisphosphonates
Two different BPs, alendronate and risedronate, are currently approved and marketed for osteoporosis prevention and treatment. A third agent, ibandronate, is currently being studied in a once-monthly regimen, but it is not as yet in clinical use.
Alendronate treatment has demonstrated a decrease in bone turnover and an increase in bone mass up to 8% versus placebo at the vertebral level and 6% versus placebo in the hip over three years in several clinical trials (Liberman et al 1995; Cranney et al 2002). More importantly, alendronate reduces the occurrence of vertebral fracture and height loss. The Fracture Intervention Trial (FIT) has provided evidence in over 2000 women with prevalent vertebral fractures (FIT 1) that daily alendronate treatment reduces vertebral fractures risk by about 50%, multiple vertebral fractures by up to 90%, and hip fractures by up to 50% (Liberman et al 1995; Black et al 1996). A later study in women with low bone density showed reduction in incidence (47%) of all nonvertebral fractures (Pols et al 1999). The weekly alendronate treatment (70 mg) has shown the same antifracture efficacy of the daily regimen.
Risedronate is another potent bisphosphonate that produces very positive effects on bone mass and bone turnover, with lumbar spine bone mass increases up to 7% versus placebo and trochanteric bone mass increases of up to 4% versus placebo over 3 years (Harris et al 1999; Reginster et al 2000). Furthermore, risedronate has been shown to prevent bone loss and to preserve trabecular architecture in early postmenopausal (age 52 years) women (Dufresne et al 2003). This molecule has been shown to reduce the risk of vertebral and nonvertebral fractures early in the course of therapy (within 6 months of initiation) in postmenopausal women (Harrington et al 2004; Roux et al 2004) with sustained protection through up to 7 years of therapy in several large clinical trials (Harris et al 1999; Reginster et al 2000; McClung et al 2001;Sorensen et al 2003): Vertebral Efficacy with Risedronate Therapy–North America (VERT1), Vertebral Efficacy with Risedronate Therapy–Multinational (VERT2), and the Hip Intervention Program (HIP).
The VERT studies (over 3000 women with established osteoporosis) indicate that 3 years’ risedronate treatment reduces the risk of vertebral fractures by 40% to 50%. (Harris et al 1999; Reginster et al 2000), while the HIP study (the largest osteoporosis treatment trial ever performed) was designed to evaluate hip fracture efficacy of risedronate (McClung et al 2001; Sorensen et al 2003).
Ibandronate is a potent N-BP, which has been extensively studied in clinical development for its potential to be administered less frequently than at weekly intervals. Uniquely, ibandronate can be administered either orally or intravenously, with an interval of >2 months. Oral ibandronate, when administered either daily or intermittently, demonstrates important antifracture efficacy at the spine and hip (50%–60% risk reduction versus placebo), accompanied by significant increases in BMD at the spine and hip, and suppression of bone turnover markers in postmenopausal women (Tankó et al 2003; Chesnut et al 2004; McClung et al 2004; Reginster 2005). Studies of intermittent intravenous ibandronate in postmenopausal osteoporotic women have shown a dose-related increase in BMD and bone turnover marker suppression comparable with those obtained with the proven effective oral ibandronate regimen. In these trials, the oral and intravenous ibandronate regimens were well tolerated. Ongoing large multinational clinical trials are investigating two intermittent ibandronate regimens (once-monthly oral and intermittent intravenous injections), which are expected to provide an optimal combination of efficacy, tolerability, and patient convenience, leading to improved treatment adherence (Tankó et al 2003; Chesnut et al 2004; McClung et al 2004; Reginster 2005).
The optimal duration of therapy with BPs is still unknown but since they are retained over time in the skeleton and may exert long-term effects, a 5–7 year course is probably safe and effective on the basis of clinical trial data. Moreover, a safe clinical choice might be in aged patients or in patients with a high turnover.
Bisphosphonates must be taken with water and on an empty stomach to avoid interference with drug absorption since it is poorly absorbed. BPs are contraindicated in patients who have esophageal stricture or inadequate emptying of the esophagus due to the potential for esophageal irritation. Although there was no increased risk in clinical trials, patients are recommended to remain upright after taking the medication for at least 30 minutes and until after the first food of the day. Safety profiles appear significantly improved with the once-weekly dosing of alendronate which, as already mentioned, is therapeutically equivalent to daily dosing (Bauer et al 2000; Schnitzer et al 2000).
Mechanism of action of SERMs
Endogenous 17β–estradiol exerts a protective effect on bone; for this reason estrogen replacement therapy (ERT) has been reported to be clinically useful in preventing bone loss in postmenopausal osteoporosis (Lindsay 1976; Kalu et al 1991). Unfortunately, long-term ERT is associated with a number of undesirable side effects, including an increased risk of uterine and breast cancer and cardiovascular events (Barrett-Connor 1992; Compston 1992; Colditz et al 1995; Cauley et al 1996; WHI 2002). Therefore, there has been the need for a “ideal” compound mimicking the beneficial effects of estrogen on skeletal tissue, without producing the adverse effects of long-ERT on reproductive tissues (Sahiner et al 1998). SERMs describe a new class of estrogen receptor-binding chemicals (Sato 1992, McDonnell 2000) that exert estrogen-agonistic effects in some target tissue such as bone and lipid metabolism and estrogen-antagonistic effects on uterine endometrium and breast tissue (Ravnikar 1992; Kauffman and Bryant 1995; Ke et al 1995; Yang et al 1996; Delmas et al 1997; Walsh et al 1998; Cummings et al 1999).
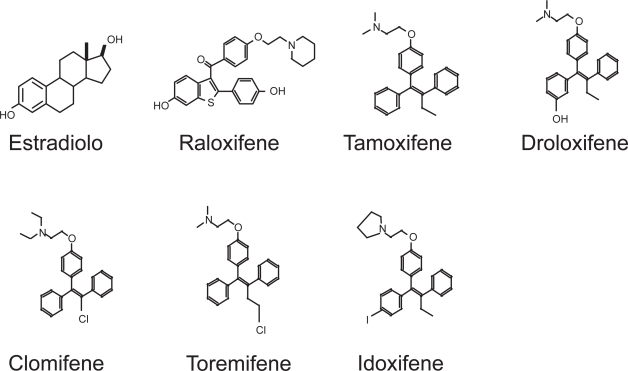
Clinically available SERMs fall into two chemical classes: triphenylethylenes and benzothiofenes (Figure 3). Tamoxifen, a triphenylethylene, belongs to the first generation of SERM, exhibits antagonist activity in mammary tissue (Short et al 1996) and produces estrogen agonist effects on bone (Powles et al 1996) and lipids (Decensi et al 1998); however tamoxifen produces an estrogen-like stimulation of the uterus in ovariectomized rats (Sato and Bryant 1996) and only partially antagonizes estrogen-induced uterine stimulation (Bryant et al 1995). For this reason, tamoxifen’s only clinical application is as chemotherapetical option in oncologic patients affected by estrogen receptor-positive breast cancer.
Figure 3.
Chemical structures of estradiol and some SERMs.
Abbreviations: SERMs, selective estrogen receptor modulators.
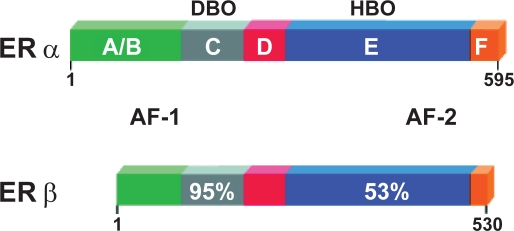
The partial agonist effect of tamoxifen on the uterus (Tomas et al 1995) is completely abolished in the newly developed second generation of SERM, Raloxifene (RAL). RAL, a benzothiophene, was developed specifically to avoid the uterotrophic effects of other SERMs, and is currently approved for prevention and treatment of postmenopausal osteoporosis (Riggs and Hartmann 2003). RAL exerts its effects by the binding with the estrogen receptor (ER). Bone cells express two estrogen receptors, α and β (Vidal et al 1999), which belong to the large nuclear steroid/thyroid hormone receptor family of ligand-dependent transcription factors (Evans 1988. Like other member of the superfamily ERs have several functional domains: the N-terminal A/B domain containing the ligand-independent transcription activation function-1 (AF-1), the C (DNA-binding) domain, the D (hinge) domain, and the C-terminal E/F (ligand-binding) domain containing the ligand-dependent transcription activation function-2 (AF- 2) (Nilsson and Gustafsson 2002; Kumar and Thompson 1999) (Figure 4).
Figure 4.
The domain structure of the oestrogen receptors. The N-terminal domain with its ligand-independent activation function-1 (AF-1). The C (DNA-binding) domain mediates sequence-specific DNA binding. The D- or hinge-domain contains nuclear translocation signal and the multifunctional E/F domain, responsible for ligand binding, homo- and heterodimerization, and ligand-dependent co-factor interaction (AF-2).
Estrogens diffuse into cells and binds to the ERs, triggering ER dimerization as well as a specific conformational change (Jensen 1991). This change facilitates binding of coregulatory proteins that modify and activate its transcriptional activity on specific consensus ER response elements in the promoter regions of target genes. Analyses of the molecular structure of ERα crystals complexed to raloxifene or estradiol have confirmed that raloxifene and estrogen interact with the ligand-binding domain, with minor conformational differences (Brzozowski et al 1997).
RAL, acting on bone as estrogen, modulates skeletal homeostasis by decreasing bone remodeling to the premenopausal range, diminishing activity of osteoclasts, but at the same time maintaining the physiological function of osteoblasts (Taranta et al 2002). RAL, by a direct ER-dependent mechanism, decreases osteoclast differentiation from hematopoietic precursors, without increasing cell apoptosis. At the same time, RAL decreases osteoclast activity, as demonstrated by the decrease on bone resorptive activity of osteoclast in an in vitro experimental bone resorption assay (Taranta et al 2002). Interestingly, while BPs directly act on osteoclasts, RAL appears to act by two different and independent mechanisms: a direct action on the bone resorbing cell, but also an osteoblast-mediated mechanism. Indeed, in vitro data demonstrated that RAL inhibits release of cytokines, as interleukin-6 and tumor necrosis factor-α(TNF-α) from osteoblasts (Taranta et al 2002). Additionally, this molecule also interferes with OPG/RANKL/RANK cytokine system, essential for osteoclast biology, which strongly suggests that this SERM can modulate osteoclast activity by a paracrine osteoblast-dependent mechanism (Hofbauer et al 2004). Interestingly, RAL increased the osteoblast-specific transcription factor Cbfa1/Runx2 and α2 procollagen type I chain mRNAs, with a pattern that partially coincided with that of 17β-estradiol (Taranta et al 2002), strongly suggesting that this molecule could partially act on bone metabolism not fully blocking bone remodeling, but instead modulating it as estrogens do before menopause. More interestingly, our recent studies have demonstrated that RAL treatment is able to decrease circulating cytokines levels in postmenopausal osteoporotic women (Gianni et al 2004), strongly suggesting that this molecule could modulate bone turnover by controlling cytokines levels after menopause.
RAL can be defined an antiresorptive agent which blunts the excessive bone resorption occurring in the high bone turnover state induced by estrogen deficiency after the menopause.
Clinical use of SERMs
In a large treatment study, over 7700 women with osteoporosis were randomly assigned to receive two different doses of RAL versus placebo in addition to calcium and vitamin D (Ettinger et al 1999). RAL reduced the occurrence of vertebral fracture by 30% to 50%; however, in this trial and the 1-year extension, there were no suggestions of reduced risk of any other osteoporotic fracture. However, this study was not specifically designed, nor did it have the adequate power to evaluate the risk of other fractures. To fully address this point, a large, randomized, controlled trial called Raloxifene Use for the Heart (RUTH) is under way, which will evaluate nonspine fractures as one of its many outcomes.
Raloxifene, like tamoxifen and estrogen, has positive effects on other organ systems beside the skeleton in postmenopausal women (Fisher et al 1998; Ettinger et al 1999; Delmas et al 2002; Rossouw et al 2002). One positive effect appears to be a reduction in estrogen receptor-positive invasive breast cancer occurrence of about 65% over 4 years in women taking raloxifene compared with placebo (Cauley et al 2001). The persistence of this effect is still under investigation in the RUTH study. Additionally, Raloxifene reduces serum total and low-density lipoprotein cholesterol, lipoprotein (a), and fibrinogen. In a recent publication in which cardiovascular disease outcomes were evaluated in a secondary analysis of participants in the Multiple Outcomes of Raloxifene Evaluation (MORE) trial, RAL had no significant effect on cardiovascular events in the whole study population. However, when analysis was performed on osteoporotic women with increased cardiovascular risk at baseline, those assigned to the RAL group had a significant 40% reduction in risk of incident cardiovascular events compared with placebo (Barrett-Connor et al 2002). Also this effect is under further evaluation in the RUTH study. Additionally, RAL is not associated with an increase in the risk of uterine cancer or any benign uterine disease, but may, like tamoxifen, increase the occurrence of hot flashes (Cummings et al 1999) and, similarly to tamoxifen and estrogen, increase the risk of venous thromboembolic disease (Delmas et al 2002). Cognitive-function studies from the osteoporosis treatment trial and other smaller studies indicate absence of negative effects on cognitive function and no differences in memory or mood (Nickelsen et al 1999; Yaffe et al 2001). The optimal duration of therapy with raloxifene, as with the other therapies, is still unclear, although at least 5 year treatment may be suggested and it is probably effective on the basis of clinical trial data in the prevention and treatment of postmenopausal osteoporosis. Raloxifene must be taken daily, independently of the meal, since no food interference has been shown.
Mechanism of action of bone forming agents (PTH)
Parathyroid hormone (PTH) has been recognized as an anabolic agent in bone tissue for over 70 years (Selye 1932) but, until recently, its catabolic activity in maintaining plasma calcium homeostasis received most attention. This hormone is secreted by the parathyroid glands in response to a decreased plasmatic calcium levels, acting to restore normocalcemia by increasing the efflux of calcium from bone, by increasing calcium reabsorption by the kidney and, indirectly, by increasing the renal synthesis of 1, 25-dihydroxyvitamin D, which increases intestinal calcium absorption. Whether PTH is catabolic or anabolic in the skeleton depends on the manner in which the hormone is presented to bone; sustained exposure to elevated PTH levels, as seen in hyperparathyroidism, results in bone loss whereas intermittent exposure, as occurs following PTH injection, increases bone mass. Teriparatide (1–34 PTH) is the first biosynthesized bone forming agent available for the treatment of osteoporosis. The active (1–34) appears to have the same anabolic effects on bone as PTH, 1–31 PTH, and 1–84 PTH (Rehman et al 2003). The molecule’s effects are mediated by the binding with a specific receptor in both osteoblasts and renal tubular cells (Juppner et al 1991; Usdin et al 1999). The binding of the molecule to the PTH receptor-1 activates adenylate cyclase and several phospholipases (A, C, D) and increases intracellular cAMP and calcium levels. Acting through these mechanisms all these molecules appear anabolic in respect to bone.
Clinical use of bone forming agents
Teriparatide is the only anabolic osteoporosis medication. Clinical randomized, controlled trials, conducted to evaluate the role of recombinant human parathyroid hormone (rhPTH) in postmenopausal women affected by severe osteoporosis, show that PTH is highly effective at increasing BMD in the spine and throughout the skeleton. Results from biopsy and bone turnover indicate that it works by stimulating new bone formation on trabecular, endocortical, and periosteal bone surfaces by preferentially stimulating osteoblastic activity over osteoclastic activity (Dempster et al 2001). PTH enhances bone strength by restoring bone architecture in both cancellous and cortical bone, expanding bone size as well as improving BMD. A large, multicenter, clinical trial of teriparatide in postmenopausal women showed significant increase in bone mass at the lumbar spine (9.7%), total hip (2.6%), total body bone mineral in teriparatide-treated women (Neer et al 2001) as well as a significant 65% reduction in vertebral fractures (Neer et al 2001) and a 53% reduction in nonvertebral fractures in teriparatide-treated patients (Neer et al 2001). In women with osteoporosis who have been previously treated with raloxifene or hormone therapy, it appears that PTH maintains the same efficacy obtained in untreated patients (Cosman and Lindsay 2004), while there is a suggestion that prior treatment with alendronate may blunt some of skeletal response to PTH (Ettinger et al 2004); however, BMD increases significantly; bone turnover is dramatically stimulated; and there are no head-to-head trials confirming that the BMD effect is smaller than expected in these patient groups. Moreover, animal studies indicate that even if BMD changes are smaller in response to PTH after pretreatment with a bisphosphonate, bone strength may be equally increased in the clinical setting as in the animals given PTH without prior antiresorptive agents (Ma et al 2003). Therefore, in patients who have been previously on other antiresorptive medications, PTH can and should be given without being afraid of decreased efficacy.
Additionally, due to the innovative mechanism of action of this molecule, questions have arisen whether the coadministration of teriparatide with other antiresorptive agents might have additional positive effects in terms of both BMD or fracture risk. One study suggests that there is no benefit to coadministration of PTH (the intact 1–84 molecule in this study) with alendronate over PTH alone (Black et al 2003). Thus, at this time, on the basis of the available clinical data, it is reasonable to propose that PTH be used alone in a newly diagnosed, previously untreated patient, or in osteoporotic patients previously treated with alternative therapeutical options. Additionally, all patients, after the PTH course, should be given a potent antiresorptive agent to maintain the PTH-induced increased bone mass and antifracture efficacy.
Parathyroid hormone currently must be administered by subcutaneous injection, although alternative modes of delivery are being investigated. The Food and Drug Administration (FDA) has approved the use of teriparatide for 2 years in postmenopausal women and men at high risk for fracture. It is contraindicated in patients with Paget’s disease of the bone or patients with bone metastases and preexisting hypercalcemia. The most common side effects associated with teriparatide include dizziness and leg cramps.
Additionally, clinical trials have been performed, and others are ongoing, to evaluate fracture efficacies of therapeutical regimen with association of two different antiosteoporotic drugs. One small trial of PTH with hormone therapy versus hormone therapy alone showed that PTH could dramatically reduce vertebral deformity occurrence (Cosman et al 2001). On the other hand, association between raloxifene with alendronate did not show any therapeutical advantages in the association group in comparison with the placebo group, which would indicate the lack of further advantages in the association group.
Conclusions
In conclusion, by considering all the above described differential mechanism of action and clinical studies, it might be useful to re-evaluate an approach to osteoporosis treatment which gives primary importance to a careful characterization of the patient, in terms of skeletal risk factors (ie, bone density and quality, remodelling), but also extraskeletal risk factors (ie, age, nutritional status, exercise) to optimize therapeutical approach to prevent fractures. Therefore, the first-choice drug would be the one that better meets the physician’s needs, but also the “expectations” of the patient (Del Puente et al 2004; Gandolini et al 2004).
Careful monitoring and evaluation of the presence and/or the appearance of contraindications or side effects is required, which suggests the need for a second choice drug. Moreover, it is essential to realize that the patient will change, and may develop different clinical indications over time. In addition, specific attention has to be paid to adequate nutritional intake, exercise, and hip protection in order to prevent fractures (Del Puente et al 2004). Since a wide number of treatment options are available, and others are in the process of being developed, on the base of their different mechanisms of action, it will be possible to customize the best therapeutical option for each individual patient.
The field of bone diseases is rapidly advancing both in terms of basic research and clinical investigation. Further knowledge may enrich and modify our pharmacological approach in the near future.
References
- Barrett-Connor E. Hormone replacement and cancer. Br Med Bull. 1992;48:345–55. doi: 10.1093/oxfordjournals.bmb.a072550. [DOI] [PubMed] [Google Scholar]
- Barrett-Connor E, Grady D, Sashegyi A, et al. Raloxifene and cardiovascular events in osteoporotic postmenopausal women: four-year randomized trial. JAMA. 2002;287:847–57. doi: 10.1001/jama.287.7.847. [DOI] [PubMed] [Google Scholar]
- Bauer DC, Black D, Ensrud K, et al. Upper gastrointestinal tract safety profile of alendronate. The Fracture Intervention Trial. Arch Intern Med. 2000;160:517–25. doi: 10.1001/archinte.160.4.517. [DOI] [PubMed] [Google Scholar]
- Benford HL, MC Gowan NW, Helfrich MH, et al. Visualization of bisphosphonate-induced caspase-3 activity in apoptotic osteoclasts in vitro. Bone. 2001;28:465–73. doi: 10.1016/s8756-3282(01)00412-4. [DOI] [PubMed] [Google Scholar]
- Bjarnason NH, Sarkar S, Duong T, et al. Six and twelve month changes in bone turnover are related to reduction in vertebral fracture risk during 3 years of raloxifene treatment in postmenopausal osteoporosis. Osteoporos Int. 2001;12:922–30. doi: 10.1007/s001980170020. [DOI] [PubMed] [Google Scholar]
- Black D, Cummings S, Karpf D, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet. 1996;348:1535–41. doi: 10.1016/s0140-6736(96)07088-2. [DOI] [PubMed] [Google Scholar]
- Black DM, Greenspan SL, Ensrud KE, et al. The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med. 2003;349:1207–15. doi: 10.1056/NEJMoa031975. [DOI] [PubMed] [Google Scholar]
- Boivin G, Meunier PJ. The mineralization of bone tissue: a forgotten dimension in osteoporosis research. Osteoporos Int. 2003;3:S19–24. doi: 10.1007/s00198-002-1347-2. [DOI] [PubMed] [Google Scholar]
- Boyce RW, Paddock CL, Gleason JR, et al. The effect of risedronate on canine cancellous bone remodeling: three dimensional kinetic reconstruction of the remodeling site. J Bone Miner Res. 1995;10:211–21. doi: 10.1002/jbmr.5650100207. [DOI] [PubMed] [Google Scholar]
- Bryant HU, Glasebrook AL, Yang NN, et al. A pharmacological review of raloxifene. J Bone Miner Metab. 1995;14:1–9. [Google Scholar]
- Brzozowski AM, Pike ACW, Dauter Z, et al. Molecular basis of agonism and antagonism in the oestrogen receptor. Nature. 1997;389:753–8. doi: 10.1038/39645. [DOI] [PubMed] [Google Scholar]
- Burgess TL, Quian Y, Kaufman S, et al. The ligand for osteoprotegerin (OPGL) directly activates mature osteoclasts. J Cell Biol. 1999;145:527–38. doi: 10.1083/jcb.145.3.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauley JA, Lucas FL, Kuller FH, et al. Bone mineral density and risk of breast cancer in older women: the study of osteoporotic fractures. JAMA. 1996;276:1404–8. [PubMed] [Google Scholar]
- Cauley JA, Norton L, Lippman ME, et al. Continued breast cancer risk reduction in postmenopausal women treated with raloxifene: 4-year results from the MORE trial. Breast Cancer Rest Treatment. 2001;65:124–34. doi: 10.1023/a:1006478317173. [DOI] [PubMed] [Google Scholar]
- Cenci S, Toraldo G, Weitzmann MN, et al. Estrogen deficiency induces bone loss by increasing T cell proliferation and lifespan through IFN-γ-induced class II transactivator. PNAS USA. 2003;2:10405–10. doi: 10.1073/pnas.1533207100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesnut CH, Skag A, Christiansen C, et al. Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res. 2004;19:1241–9. doi: 10.1359/JBMR.040325. [DOI] [PubMed] [Google Scholar]
- Chesnut CH, Silverman S, Andriano K, et al. A randomized trial of nasal spray salmon calcitonin in postmenopausal women with established osteoporosis: the prevent recurrence of osteoporotic fractures study. PROOF Study Group. Am J Med. 2000;109:267–76. doi: 10.1016/s0002-9343(00)00490-3. [DOI] [PubMed] [Google Scholar]
- Colditz GA, Hankinson SE, Hunter DJ, et al. The use of estrogens and progestins and the risk of breast cancer in postmenopausal women. N Engl J Med. 1995;332:1589–93. doi: 10.1056/NEJM199506153322401. [DOI] [PubMed] [Google Scholar]
- Compston JE. HRT and osteoporosis. Br Med Bull. 1992;48:309–14. doi: 10.1093/oxfordjournals.bmb.a072549. [DOI] [PubMed] [Google Scholar]
- Cosman F, Nieves J, Woelfert L, et al. Parathyroid hormone added to established hormone therapy: effects on vertebral fracture and maintenance of bone mass after PTH withdrawal. J Bone Miner Res. 2001;16:925–31. doi: 10.1359/jbmr.2001.16.5.925. [DOI] [PubMed] [Google Scholar]
- Cosman F, Lindsay R. Therapeutic potential of parathyroid hormone. Curr Osteoporos Rep. 2004;2:5–11. doi: 10.1007/s11914-004-0008-0. [DOI] [PubMed] [Google Scholar]
- Cranney A, Wells G, Willan A, et al. Meta-analyses of therapies for postmenopausal osteoporosis. II. Meta-analysis of alendronate for the treatment of postmenopausal women. Endocr Rev. 2002;23:508–16. doi: 10.1210/er.2001-2002. [DOI] [PubMed] [Google Scholar]
- Cummings SR, Eckert S, Krueger KA, et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. JAMA. 1999;281:2189–97. doi: 10.1001/jama.281.23.2189. [DOI] [PubMed] [Google Scholar]
- Decensi A, Bonanni B, Guerrieri-Gonzaga A, et al. Biologic activity of tamoxifen at low doses in healthy women. J Natl Cancer Inst. 1998;90:1461–7. doi: 10.1093/jnci/90.19.1461. [DOI] [PubMed] [Google Scholar]
- Delmas PD, Bjarnason NH, Mitlak BH, et al. Effects of raloxifene on bone mineral density, serum cholesterol concentrations, and uterine endometrium in postmenopausal women. N Engl J Med. 1997;337:1641–7. doi: 10.1056/NEJM199712043372301. [DOI] [PubMed] [Google Scholar]
- Delmas PD, Ensrud KE, Adachi JD, et al. Efficacy of raloxifene on vertebral fracture risk reduction in postmenopausal women with osteoporosis: four-year results from a randomized clinical trial. J Clin Endocrinol Metab. 2002;87:3609–17. doi: 10.1210/jcem.87.8.8750. [DOI] [PubMed] [Google Scholar]
- Del Puente A, Migliaccio S, Esposito A, et al. A reappraisal of therapeutic approaches to osteoporosis. Aging Clin Exp Res. 2004;3:S42–S46. [PubMed] [Google Scholar]
- Dempster DW, Cosman F, Kurland ES, et al. Effects of daily treatment with parathyroid hormone on bone microarchitecture and turnover in patients with osteoporosis: a paired biopsy study. J Bone Miner Res. 2001;16:1846–53. doi: 10.1359/jbmr.2001.16.10.1846. [DOI] [PubMed] [Google Scholar]
- Diez-Perez A. Bisphosphonates. Maturitas. 2002;43(Suppl):S19–S26. doi: 10.1016/s0378-5122(02)00145-7. [DOI] [PubMed] [Google Scholar]
- Dufresne E, Chmielewski PA, Manhart MD, et al. Risedronate preserves bone architecture in early postmenopausal women in 1 year as measured by three-dimensional microcomputed tomography. Calcif Tissue Int. 2003;73:423–32. doi: 10.1007/s00223-002-2104-4. [DOI] [PubMed] [Google Scholar]
- Ettinger B, Black DM, Mitlak BH, et al. Reduction of vertebral fracture risk in post menopausal women with osteoporosis treated with raloxifene. Results from a 3-year randomized clinical trial. JAMA. 1999;282:637–45. doi: 10.1001/jama.282.7.637. [DOI] [PubMed] [Google Scholar]
- Ettinger B, San Martin J, Crans G, et al. Differential effects of teriparatide on BMD after treatment with raloxifene or alendronate. J Bone Miner Res. 2004;19:745–51. doi: 10.1359/JBMR.040117. [DOI] [PubMed] [Google Scholar]
- Evans RM. The steroid and thyroid hormone superfamily. Science. 1988;240:889–95. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher B, Costantino JP, Wickerham DL, et al. Tamoxifen for prevention of breast cancer. Report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–88. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- Tankó LB, Felsenberg D, Czerwiski E, et al. Oral weekly ibandronate prevents bone loss in postmenopausal women. J Intern Med. 2003;254:159–67. doi: 10.1046/j.1365-2796.2003.01174.x. [DOI] [PubMed] [Google Scholar]
- Gandolini G, Migliaccio S, Bevilacqua M, et al. Prevent, treat and maintain: a new goal for osteoporosis management in clinical practice. Aging Clin Exp Res. 2004;3:S37–S41. [PubMed] [Google Scholar]
- Gianni W, Ricci A, Gazzaniga P, et al. Raloxifene modulates interleukin-6 and tumor necrosis factor-alpha synthesis in vivo: results from a pilot clinical study. J Clin Endocrinol Metab. 2004;12:6097–9. doi: 10.1210/jc.2004-0795. [DOI] [PubMed] [Google Scholar]
- Gold DT. The nonskeletal consequences of osteoporotic fractures. Psychologic and social outcomes. Rheum Dis Clin North Am. 2001;27:255–62. doi: 10.1016/s0889-857x(05)70197-6. [DOI] [PubMed] [Google Scholar]
- Harris ST, Watts NB, Genant HK, et al. Effect of risedronate treatment on vertebral and non vertebral fractures in women with postmenopausal osteoporosis. A randomized controlled trial. JAMA. 1999;282:1344–52. doi: 10.1001/jama.282.14.1344. [DOI] [PubMed] [Google Scholar]
- Harrington JT, Ste-Marie LG, Brandi ML, et al. Risedronate rapidly reduces the risk for nonvertebral fractures in women with postmenopausal osteoporosis. Calcif Tissue Int. 2004;74:129–35. doi: 10.1007/s00223-003-0042-4. [DOI] [PubMed] [Google Scholar]
- Hodsman AB, Bauer DC, Dempster DW, et al. Parathyroid hormone and teriparatide for the treatment of osteoporosis: a review of the evidence and suggested guidelines for its use. Endocr Rev. 2005;26:688–7. doi: 10.1210/er.2004-0006. [DOI] [PubMed] [Google Scholar]
- Hofbauer LC, Kuhne CA, Viereck V. The OPG/RANKL/RANK system in metabolic bone diseases. J Musculoskelet Neuronal Interact. 2004;4:268–75. [PubMed] [Google Scholar]
- Hughes DE, Dai A, Tiffee JC, et al. Estrogen promotes apoptosis of murine osteoclasts mediated by TGF-beta. Nat Med. 1996;2:1132–6. doi: 10.1038/nm1096-1132. [DOI] [PubMed] [Google Scholar]
- Jensen EV. Overview of the nuclear receptor family. In: Parker MG, editor. Nuclear hormone receptors: molecular mechanisms, cellular functions, clinical abnormalities. London: Acad Pr; 1991. pp. 1–13. [Google Scholar]
- Jilka RL, Hangoe G, Girasole G, et al. Increased osteoclast development after estrogen loss: mediation by interleukin-6. Science. 1992;257:88–91. doi: 10.1126/science.1621100. [DOI] [PubMed] [Google Scholar]
- Juppner H, Hesch RD. Biochemical characterization of cellular hormone receptors. Curr Top Pathol. 1991;83:53–69. doi: 10.1007/978-3-642-75515-6_3. [DOI] [PubMed] [Google Scholar]
- Kado DM, Browner WS, Palermo L, et al. Vertebral fractures and mortality in older women: a prospective study. Study of Osteoporotic Fractures Research Group. Arch Intern Med. 1999;159:1215–20. doi: 10.1001/archinte.159.11.1215. [DOI] [PubMed] [Google Scholar]
- Kalu DN, Liu CC, Salerno E, et al. Skeletal response of ovariectomized rats to low and high doses of 17 beta-estradiol. J Bone Miner Res. 1991;14:175–87. doi: 10.1016/0169-6009(91)90021-q. [DOI] [PubMed] [Google Scholar]
- Kauffman RF, Bryant HU. Selective estrogen receptor modulators. Drug News Perspect. 1995;8:531–8. [Google Scholar]
- Ke HZ, Simmons HA, Piries CM, et al. Droloxifene, a new estrogen antagonist/agonist, prevents bone loss in ovariectomized rats. Endocrinology. 1995;136:2435–41. doi: 10.1210/endo.136.6.7750465. [DOI] [PubMed] [Google Scholar]
- Klotzbuecher CM, Ross PD, Landsman PB, et al. Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res. 2000;15:721–39. doi: 10.1359/jbmr.2000.15.4.721. [DOI] [PubMed] [Google Scholar]
- Kumar R, Thompson EB. The structure of the nuclear hormone receptors. Steroids. 1999;64:310–19. doi: 10.1016/s0039-128x(99)00014-8. [DOI] [PubMed] [Google Scholar]
- Liberman UA, Weiss SR, Broll J, et al. Effect of alendronate on BMD and the incidence of fractures in postmenopausal osteoporosis. N Engl J Med. 1995;36:612–13. doi: 10.1056/NEJM199511303332201. [DOI] [PubMed] [Google Scholar]
- Lindsay R, Hart DM, Aitken JM, et al. Long-term prevention of post-menopausal osteoporosis by oestrogen. Evidence for an increased bone mass after delayed onset of oestrogen treatment. Lancet. 1976;1:1038–41. doi: 10.1016/s0140-6736(76)92217-0. [DOI] [PubMed] [Google Scholar]
- Lindsay R, Silverman SL, Cooper C, et al. Risk of new vertebral fracture in the year following a fracture. JAMA. 2001;285:320–3. doi: 10.1001/jama.285.3.320. [DOI] [PubMed] [Google Scholar]
- Ma YL, Bryant HU, Zeng Q, et al. New bone formation with teriparatide [human parathyroid hormone-(1–34)] is not retarded by long-term pretreatment with alendronate, estrogen, or raloxifene in ovariectomized rats. Endocrinology. 2003;144:2008–15. doi: 10.1210/en.2002-221061. [DOI] [PubMed] [Google Scholar]
- Manolagas C. Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev. 2000;21:115–37. doi: 10.1210/edrv.21.2.0395. [DOI] [PubMed] [Google Scholar]
- McClung MR, Geusens P, Miller PD, et al. Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group. N Engl J Med. 2001;344:333–40. doi: 10.1056/NEJM200102013440503. [DOI] [PubMed] [Google Scholar]
- McClung MR, Wasnich RD, Recker R, et al. Oral daily ibandronate prevents bone loss in early postmenopausal women without osteoporosis. J Bone Miner Res. 2004;19:11–18. doi: 10.1359/JBMR.0301202. [DOI] [PubMed] [Google Scholar]
- McDonnell DP, Wijayaratne A, Chang C, et al. Elucidation of the molecular mechanism of action of selective estrogen receptor modulators. Am J Cardiol. 2002;90:F35–F43. doi: 10.1016/s0002-9149(01)02221-4. [DOI] [PubMed] [Google Scholar]
- Migliaccio S, Falcone S, Spera G. Bone modeling and remodeling: from biology to clinical application. Aging Clin Exp Res. 2004;3:S20–S22. [PubMed] [Google Scholar]
- Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1–34) on fractures and bone mineral density in post-menopausal women with osteoporosis. N Engl J Med. 2001;344:1434–41. doi: 10.1056/NEJM200105103441904. [DOI] [PubMed] [Google Scholar]
- Nickelsen T, Lifkin EG, Riggs BL, et al. Raloxifene hydrochloride, a selective estrogen receptor modulator: safety assessment of effects on cognitive function and mood in postmenopausal women. Psychon euroendocrinology. 1999;24:115–28. doi: 10.1016/s0306-4530(98)00041-9. [DOI] [PubMed] [Google Scholar]
- [NIH] NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy Osteoporosis prevention, diagnosis, and therapy. JAMA. 2001;285:785–95. doi: 10.1001/jama.285.6.785. [DOI] [PubMed] [Google Scholar]
- Nilsson S, Gustafsson JÅ. Structure and function of the estrogen receptor. In: Manni A, Verderame MF, editors. Contemporary endocrinology: Selective estrogen receptor modulators: Research and clinical applications. Totowa, NJ: Humana Pr; 2002. pp. 3–18. [Google Scholar]
- Pacifici R, Brown C, Puscheck E, et al. Effect of surgical menopause and estrogen replacement on cytokine release from human blood mononuclear cells. Proc Natl Acad Sci USA. 1991;88:5134–8. doi: 10.1073/pnas.88.12.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pols HA, Felsenberg D, Hanley DA, et al. Multinational, placebo-controlled randomized trial of the effects of alendronate on bone density and fracture risk in postmenopausal women with low bone mass: results of the FOSIT study. Foxamax International Trial Study Group. Osteoporos Int. 1999;9:461–8. doi: 10.1007/pl00004171. [DOI] [PubMed] [Google Scholar]
- Powles TJ, Hickish T, Kanis JA, et al. Effect of tamoxifen on bone mineral density measured by dual-energy x-ray absorptiometry in healthy premenopausal and postmenopausal women. J Clin Oncol. 1996;14:78–84. doi: 10.1200/JCO.1996.14.1.78. [DOI] [PubMed] [Google Scholar]
- Raisz LG. Local and systemic factors in the pathogenesis of osteoporosis. World Rev Nutr Diet. 1993;72:92–101. doi: 10.1159/000422331. [DOI] [PubMed] [Google Scholar]
- Raisz LG. Pathogenesis of postmenopausal osteoporosis. Rev Endocr Metab Disord. 2001;2:5–12. doi: 10.1023/a:1010074422268. [DOI] [PubMed] [Google Scholar]
- Ravnikar VA. Compliance with hormone replacement therapy: are women receiving the full impact of hormone replacement therapy preventative health benefits? Womens Health Issues. 1992;2:75–82. doi: 10.1016/s1049-3867(05)80275-0. [DOI] [PubMed] [Google Scholar]
- Reginster JY, Minne H, Sorensen O, et al. Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Osteoporos Int. 2000;11:83–91. doi: 10.1007/s001980050010. [DOI] [PubMed] [Google Scholar]
- Reginster JY. Ibandronate oral and intravenous ibandronate in the management of postmenopausal osteoporosis: a comprehensive review. Curr Pharm Des. 2005;11:3711–28. doi: 10.2174/138161205774580750. [DOI] [PubMed] [Google Scholar]
- Rehman Q, Lang TF, Arnaud CD, et al. Daily treatment with parathyroid hormone is associated with an increase in vertebral cross-sectional area in postmenopausal women with glucocorticoid-induced osteoporosis. Osteoporos Int. 2003;14:77–81. doi: 10.1007/s00198-002-1312-0. [DOI] [PubMed] [Google Scholar]
- Reszka AA, Rodan GA. Nitrogen-containing bisphosphonate mechanism of action. Mini Rev Med Chem. 2004;4:711–19. [PubMed] [Google Scholar]
- Riggs BL, Hartmann LC. Selective estrogen-receptor modulators—Mechanism of action and application to clinical practice. New Engl J Med. 2003;348:618–29. doi: 10.1056/NEJMra022219. [DOI] [PubMed] [Google Scholar]
- Roschger P, Rinnerthaler P, Yates J, et al. Alendronate increases degree and uniformity of mineralization in cancellous bone and decreases the porosity in cortical bone of osteoporotic women. Bone. 2001;29:185–91. doi: 10.1016/s8756-3282(01)00485-9. [DOI] [PubMed] [Google Scholar]
- Rossouw JE, Anderson GL, Kooperber C, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. Principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- Roux C, Seeman E, Eastell R, et al. Efficacy of risedronate on clinical vertebral fractures within six months. Curr Med Res Opin. 2004;20:433–9. doi: 10.1185/030079903125003125. [DOI] [PubMed] [Google Scholar]
- Sahiner T, Aktan E, Kaleli B, et al. The effects of postmenopausal hormone replacement therapy on sympathetic skin response. Maturitas. 1998;30:85–8. doi: 10.1016/s0378-5122(98)00049-8. [DOI] [PubMed] [Google Scholar]
- Sato M, Rippy MK, Bryant HU. Raloxifene, tamoxifen, nafoxidine, or estrogen effects on reproductive and nonreproductive tissues in ovariectomized rats. FASEB J. 1996;10:905–12. doi: 10.1096/fasebj.10.8.8666168. [DOI] [PubMed] [Google Scholar]
- Schnitzer T, Bone HG, Crepaldi G, et al. Therapeutic equivalence of alendronate 70 mg once-weekly and alendronate 10 mg daily in the treatment of osteoporosis. Alendronate Once-Weekly Study Group. Aging (Milano) 2000;12:1–12. [PubMed] [Google Scholar]
- Seeman E. Reduced bone formation and increased bone resorption: rational targets for the treatment of osteoporosis. Osteoporos Int. a. 2003;14:S2–S8. doi: 10.1007/s00198-002-1340-9. [DOI] [PubMed] [Google Scholar]
- Seeman E.b. 2003Bone quality Osteoporos Int 14S5:3–7. [DOI] [PubMed] [Google Scholar]
- Selye H. On the stimulation of new bone formation with parathyroid extract and irradiated ergosterol. Endocrinology. 1932;16:547–58. [Google Scholar]
- Short LL, Glasebrook AL, Adrian MD, et al. Distinct effects of selective estrogen receptor modulators on estrogen dependent and estrogen independent human breast cancer cell proliferation. J Bone Miner Res. 1996;11:1. [Google Scholar]
- Sorensen OH, Crawford GM, Mulder H, et al. Long-term efficacy of risedronate: a 5-year placebo-controlled clinical experience. Bone. 2003;32:120–26. doi: 10.1016/s8756-3282(02)00946-8. [DOI] [PubMed] [Google Scholar]
- Taranta A, Brama M, Teti A, et al. The selective estrogen receptor modulator Raloxifene modulates osteoblast and osteoclast activity in vitro. Bone. 2002;30:365–37. doi: 10.1016/s8756-3282(01)00685-8. [DOI] [PubMed] [Google Scholar]
- Tomas E, Kauppila A, Blanco G, et al. Comparison between the effects of tamoxifen and toremifene on the uterus in postmenopausal breast cancer patients. Gynecol Oncol. 1995;59:261–6. doi: 10.1006/gyno.1995.0019. [DOI] [PubMed] [Google Scholar]
- Usdin TB, Hilton J, Vertesi T, et al. Distribution of the parathyroid hormone 2 receptor in rat: immunolocalization reveals expression by several endocrine cells. Endocrinology. 1999;140:3363–71. doi: 10.1210/endo.140.7.6855. [DOI] [PubMed] [Google Scholar]
- Vidal O, Kindblom LG, Ohlsson C. Expression and localization of estrogen receptor-beta in murine and human bone. J Bone Miner Res. 1999;14:923–9. doi: 10.1359/jbmr.1999.14.6.923. [DOI] [PubMed] [Google Scholar]
- Walsh BW, Kuller LH, Wild RA, et al. Effects of raloxifene on serum lipids and coagulation factors in healthy postmenopausal women. JAMA. 1998;279:1445–51. doi: 10.1001/jama.279.18.1445. [DOI] [PubMed] [Google Scholar]
- [WHI] Writing Group for the Women’s Health Initiative Investigators Risks and Benefits of Estrogen plus Progestin in Healthy Postmenopausal Women—Principal Results From the Women’s Health Initiative Randomized Controlled Trial. JAMA. 2002;288:321–33. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- Yaffe K, Krueger K, Sarkar S, et al. Cognitive function in postmenopausal women treated with raloxifene. N Engl J Med. 2001;344:1207–13. doi: 10.1056/NEJM200104193441604. [DOI] [PubMed] [Google Scholar]
- Yang NN, Venugopalan M, Hardikar S, et al. Identification of an estrogen response element activated by metabolites of 17b-estradiol and raloxifene. Science. 1996;273:1222–5. doi: 10.1126/science.273.5279.1222. [DOI] [PubMed] [Google Scholar]
- Yasuda H, Shima N, Nakagawa N, et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA. 1998;95:3597–602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]