Abstract
AIM: To study the effect of celecoxib (CXB) on diethylnitrosamine activation through the regulation of cytochrome P450 in a hepatocarcinogenesis model.
METHODS: Six-week-old male Sprague-Dawley rats were randomly divided into five groups, a non-treated group (NT), a diethylnitrosamine-treated group (DEN), a DEN+CXB-treated group (DEN+CXB), and CXB 8 d-treated and CXB 32 d-treated groups. The effects of celecoxib on the enzymatic activities of CYP1A1, 2A, 2B1/2, and 2E1 were assessed in hepatic microsomes 24 h after DEN administration. Changes in CYP1A1 and CYP2B1/2 protein expression were also evaluated. The rate of DEN metabolism was measured by the production of the deethylation metabolite acetaldehyde, and the denitrosation metabolite nitrite.
RESULTS: DEN+CXB administration produced a significant increase in the enzymatic activities of CYP2B1/2 and 1A1, whereas it did not change the activities of CYP2A and 2E1, compared to that of the DEN group. CXB treatment for eight days did not produce a significant effect on enzymatic activity when compared to the NT group; however, when it was administered for prolonged times (CXB 32 d group), the enzymatic activities were increased in a similar pattern to those in the DEN+CXB group. The observed increase in the enzymatic activities in the DEN+CXB group was accompanied by an increase in the CYP2B1/2 protein levels; no changes were observed in the levels of CYP1A1. In vitro, CXB increased the denitrosation of DEN, a pathway of metabolic detoxification. The addition of SKF-525A, a preferential inhibitor of CYP2B, abrogated the denitrosation of DEN.
CONCLUSION: These results suggest that the mechanism of action of CXB involves enhancement of the detoxification of DEN by an increasing denitrosation via CYP2B1/2.
Keywords: Hepatocarcinogenesis, Chemoprevention, Diethylnitrosamine, Denitrosation, Celecoxib, Cytochromes P450
INTRODUCTION
Hepatocellular carcinoma (HCC) is one of the most common tumors, with about one million new cases per year worldwide. Despite progress in early diagnosis and novel therapies, the overall survival of HCC patients has not been significantly improved over the last three decades. Therefore, preventive strategies are of paramount importance and need to be actively explored in order to reduce the incidence of this disease[1].
Numerous epidemiological studies have demonstrated that long-term use of cyclooxygenase-2 (COX-2) specific inhibitors, such as celecoxib (CXB), are associated with a reduced incidence of several types of cancer[2]. Studies in rodents have shown that CXB inhibits the development of chemically induced cancers, including colon, skin, urinary bladder, and breast[3–6]. The proposed mechanisms for the effects of CXB in these models include inhibition of cell proliferation, reduction of angiogenesis and induction of apoptosis[7]. Recently, we have demonstrated that CXB acts as a chemopreventive agent against the development of preneoplastic lesions induced by diethylnitrosamine (DEN), 2-acetylaminofluorene and partial hepatectomy in the modified resistant hepatocyte (MRH) model[8]. However, the exact mechanism of action by which CXB decreases liver preneoplastic lesions remains unclear, because there was no evidence of apoptosis or of changes in COX-2 expression or PGE2 production after CXB treatment. The observed reduction in proliferation markers was not sufficient to explain the reduced number of preneoplastic lesions, thus other mechanisms must be involved in the CXB effect, probably during the initial stages of hepatocarcinogenesis.
In the MRH model, DEN bioactivation is required to produce preneoplastic lesions and subsequently HCC[9]. The metabolic activation of DEN occurs during the first hours after administration, via cytochrome P450 (CYP)-dependent α-hydroxylation, which results in an ethylating agent capable of forming DNA adducts. The CYP1A1/2, 2B, 2A1/2 and 2E1 subfamilies are the major enzymes involved in the bioactivation of DEN[9–11]. In addition to the activation reaction, a denitrosation reaction may also occur, which results in nitrite production. Nitrite formation is an alternative pathway for the formation of an alkylating intermediate, and represents a carcinogen detoxification pathway[12–14]. These two pathways of DEN metabolism could occur in parallel, and although some studies suggest that both pathways are catalyzed by the same CYP enzyme, the participation of distinct isoforms must be considered. In the absence of CYP inducers, the predominant reaction is activation; nevertheless, when specific isoforms are induced, the two mechanisms compete with each other, favoring the DEN detoxification pathway[9,14].
Since there is no information in the literature about CYP regulation by CXB as a chemopreventive mechanism, the aim of this study was to determine the effect of CXB on DEN activation by affecting CYP regulation in the MRH model. These data demonstrate that the preferential modulation of CYP2B1/2 by CXB enhances DEN detoxification, which therefore blocks the initiation of the hepatocarcinogenic process.
MATERIALS AND METHODS
Materials
DEN was purchased from Sigma Chemical Co. (St. Louis, MO). Ethoxy- and pentoxy-resorufin were purchased from Molecular Probes, Inc. (Eugene, OR). Electrophoresis reagents were purchased from Bio-Rad (Hercules, CA). The monoclonal anti-rat CYP1A1 antibody was purchased from Oxford Biochemicals Research, Inc. (Oxford, MI). The monoclonal anti-rat CYP2B1/2 antibody was kindly provided by Dr. Colin Jefcoate (University of Wisconsin-Madison, Dept. of Pharmacology, Madison, WI). The horseradish peroxidase-conjugated goat anti-mouse IgG antibody was acquired from Pierce Protein Research Products (Rockford, IL).
Experimental diet
CXB was extracted from the commercial drug Celebrex® (Pfizer, Mexico City, Mexico). The identity and purity of the molecule was above 99%, as determined by nuclear magnetic resonance analysis in the Department of Chemistry at CINVESTAV (Mexico City, Mexico). Diet 5001 containing 1500 ppm of CXB was prepared by Purina Test Diet (Richmond, IN).
Animals
Six-week-old male Sprague-Dawley rats were purchased from Harlan Industries (Mexico City, Mexico). Rats were fed ad libitum and housed in a controlled environment with a 12 h light/dark cycle, 50% relative humidity and a temperature of 21 ± 2°C. All experiments were performed according to the guidelines established by the Institutional Animal Care Committee in agreement with Mexican Official Norm NOM-062-ZOO-1999.
Experimental procedure
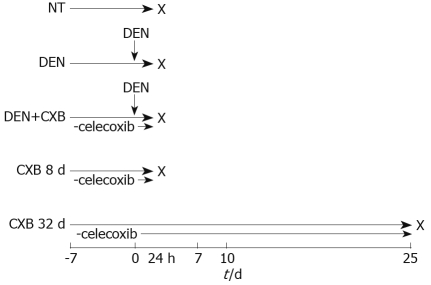
After acclimation, the rats were separated into five treatment groups (Figure 1). In the non-treated (NT) group, rats were fed with 5001 basal diet and sacrificed eight days after the beginning of the experiment; the DEN and DEN+CXB groups received a single intraperitoneal dose of DEN (200 mg/kg) and were sacrificed 24 h later. The DEN group was fed the basal diet. The DEN+CXB group was pretreated with CXB from seven days before DEN administration until sacrifice. The CXB 8 d and CXB 32 d groups were treated only with CXB for the indicated times. Animals were sacrificed by cervical dislocation and the livers were then removed and processed to obtain microsomes, as described by Mayer et al[15].
Figure 1.
Schematic representation of CXB administration in the hepatocarcinogenesis model. In the NT group, rats were maintained on a basal diet. DEN and DEN+CXB groups were treated with DEN. The CXB diet was given from 7 d before DEN administration until sacrifice, indicated with X (n = 4).
Enzymatic activities
Alkoxyresorufin metabolism assays: Microsomal O-dealkylation of 7-ethoxy-(EROD, CYP1A1) and 7-pentoxy-resorufin (PROD, CYP2B1/2) were measured fluorometrically at 37°C using 530 and 585 nm excitation and emission wavelengths, respectively[16,17].
p-Nitrophenol hydroxylase (PNPH) assay: The activity of CYP2E1 was measured by the formation of 4-nitrocatechol, which was determined spectrophotometrically at 546 nm[18].
7α-Testosterone hydroxylation: The activity of CYP2A1 was determined in microsomal suspensions obtained from treated and control rats as previously described[19]. Protein concentration was determined by Lowry’s method[20] using bovine serum albumin as a standard.
Immunoblotting
Microsomal proteins (15 and 30 μg/lane for CYP2B1/2 and CYP1A1, respectively) were separated by 10% SDS-PAGE. Proteins were blotted onto PVDF membranes. These membranes were blocked overnight at 4°C with 100 mmol/L glycine, 1% BSA and 5% non-fat dry milk in a PBS-1% Triton X-100 solution. Then, membranes were challenged with anti-rat CYP1A1 or 2B1/2 antibodies for 1 h at room temperature, followed by incubation with a horseradish peroxidase-conjugated secondary antibody for 1 h at room temperature. The specific protein bands were visualized by chemiluminescence (Santa Cruz Biotechnology, Inc.) and exposure to radiographic film. Densitometric analysis of bands was carried out using Sigma Gel software (Jandel Scientific, San Rafael, CA).
In vitro biotransformation of DEN by rat hepatic microsomes
The rate of DEN metabolism in control and CXB-treated rat hepatic microsomes was measured by the production of both a deethylation metabolite, acetaldehyde, and a denitrosation metabolite, nitrite, as previously described[12,14,21]. The enzymatic assay was performed in a final volume of 1 mL TMP buffer (50 mmol/L Tris-HCl, 10 mmol/L MgCl2, 150 mmol/L KCl, pH 7.0) containing 0.5 mg rat hepatic microsomal protein, 1.2 mmol/L NADPH and 50 mmol/L DEN. Reactions were initiated by adding DEN and incubating at 37°C for 30 min, and were then stopped by adding 0.1 mL 25% ZnSO4 and 0.1 mL saturated Ba(OH)2 in an ice bath. Samples were vortexed and centrifuged at 5000 g for 10 min. One-hundred microliter aliquots of supernatant were used for nitrite measurements using a specific colorimetric assay kit (Cayman Chemical Co., Ann Arbor, MI), according to the manufacturer’s instructions. Acetaldehyde production was determined by HPLC in a Waters Liquid Chromatography model 600 using an Xterra C18 phase reverse column (3.9 mm × 150 mm), as previously described[21]. As a control for CYP2B1/2-specificity, these assays were carried out in the presence of 50 mmol/L SKF-525A.
Statistical analysis
Data are presented as mean ± SD. Analysis of variance and the Bonferroni test were used to assess statistical differences among the tested groups, and the level of significance was set at P < 0.05. All statistical analyses were performed using SigmaStat software version 3.1 (Systat Software, Inc., Point Richmond, CA).
RESULTS
CXB modulates the enzymatic activity of CYPs
To determine whether the chemopreventive effect of CXB is associated with changes in the enzymatic activities of some CYPs, the activities of CYP1A1, CYP2A1, CYP2B1/2 and CYP2E1 were determined 24 h after DEN administration (Table 1). Eight days of CXB treatment did not produce a significant effect on any of the evaluated enzyme activities. DEN treatment significantly decreased the CYP1A1 and CYP2A1 activities by 66% and 58%, respectively, whereas CYP2B1/2 and CYP2E1 activities were increased 3.6- and 2.5-fold, respectively, in comparison to the NT group. When CXB was administered in combination with DEN (DEN+CXB group), the CYP1A1 activity was increased 3.5-fold and the CYP2B1/2 activity was increased 9-fold over the DEN group. Compared with the NT group, the increase in CYP2B1/2 activity was 33-fold, while no significant changes were observed for CYP1A1 activity. On the other hand, the DEN+CXB treatment had no influence on the activities of CYP2E1 and CYP2A1 (Table 1). Interestingly, the prolonged treatment with CXB (CXB 32 d group) produced an increase in the majority of the enzymatic activities analyzed: CYP1A1, CYP2B1/2 and CYP2E1. These results suggest that pretreatment with CXB in combination with the administration of DEN elicited a preferential induction of CYP1A1 and 2B1/2 enzymatic activities, with the 2B1/2 isoforms induced to a greater degree.
Table 1.
Effect of celecoxib on hepatic microsomal enzyme activities in a hepatocarcinogenesis assay
| Treatment |
Alkoxyresorufin O-dealkylation activity (pmol resorufin/min per mg protein) |
Testosterone hydroxylase activity (pmol of product/min per mg protein) |
p-Nitrophenol hydroxylase activity (nmol 4-nitrocatechol/min per mg protein) |
|
| EROD (CYP1A1) | PROD (CYP2B1/2) | 7α-OHT (CYP2A1/2) | PNPH (CYP2E1) | |
| NT | 13.1 ± 0.5 | 2.0 ± 0.5 | 129.2 ± 18.9 | 0.38 ± 0.07 |
| CXB 8 d | 13.3 ± 4.3 | 4.2 ± 2.2 | ND | 0.53 ± 0.16 |
| DEN | 4.5 ± 1.3a | 7.3 ± 3.2a | 54.2 ± 19.2a | 0.97 ± 0.14a |
| DEN+CXB | 15.9 ± 4.8b | 65.9 ± 35.4ab | 66.0 ± 5.9a | 0.80 ± 0.25a |
| CXB 32 d | 54.5 ± 11.3a | 114.2 ± 23.2a | 151.5 ± 29.5 | 1.40 ± 0.44a |
Male rats were treated with a single dose of DEN (200 mg/kg) ip and sacrificed 24 h after administration (DEN and DEN+CXB groups). The CXB diet was provided 7 d before DEN treatment and until sacrifice (8 d). The CXB 8 d and CXB 32 d groups received only a CXB-containing diet.
Significantly different from the NT group;
From the DEN group, according to the Bonferroni test (P < 0.05). Values shown are the mean ± SD from n = 4. ND: Not determined.
Celecoxib induces the expression of CYP2B1/2 but not CYP1A1
To determine whether the increases observed in the enzymatic activities of CYP1A1 and 2B1/2 were related to increases in protein levels, these isoforms were analyzed by immunoblotting. In the DEN-treated group, there were no significant differences in the protein expression of CYP1A1 and CYP2B1/2 compared to the NT group. The pretreatment with CXB in the DEN+CXB group significantly increased CYP2B1/2 protein expression (2.5-fold), but it had no significant effect on CYP1A1 protein expression compared to the DEN group (Figure 2). In summary, these results show that CXB differentially affects these two isoforms; the increase in the protein expression of CYP2B1/2 suggests that the regulation might be at the transcriptional level, while in the case of CYP1A1, CXB seems to regulate only the enzymatic activity.
Figure 2.
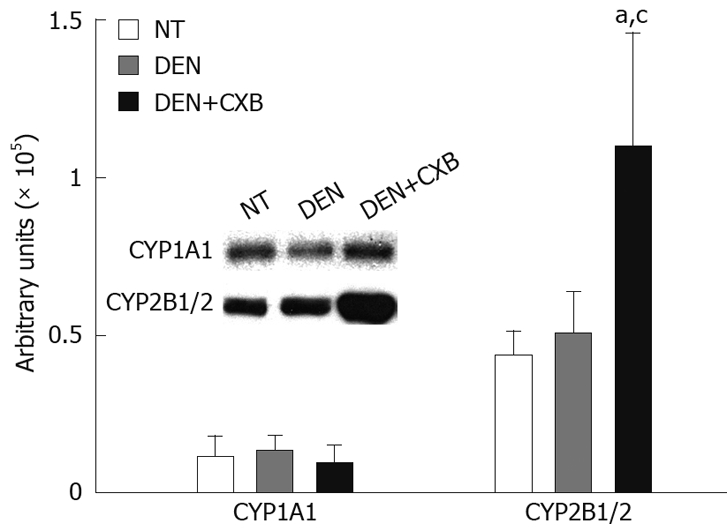
Immunodetection of hepatic microsomal CYP isoforms from DEN- and/or CXB-treated rats. Microsomal proteins (15 and 30 μg/lane for CYP2B1/2 and CYP1A1, respectively) were separated by SDS-PAGE and tested for different CYPs using specific anti-rat CYP antibodies. The bands in the inset box correspond to CYP2B1/2 and CYP1A1 protein detected in the NT, DEN and DEN+CXB groups. The graphic represents the densitometric analysis of the CYP amounts in the experimental groups. aP < 0.05, vs NT group; cP < 0.05 vs DEN group, Bonferroni test. n = 4 for all groups.
CXB favors detoxification by denitrosation of DEN
To explore whether regulation of the CYP isoforms by CXB induces the detoxification pathway of DEN as a chemopreventive mechanism, nitrite and acetaldehyde yields were measured in the microsomes of non-treated and CXB-treated rats. We used rat microsomes treated with CXB for 32 d, where the pattern of induction of enzymatic activities was similar to the pattern observed in the DEN+CXB group (with preferential induction of the 2B1/2 isoform), because the eight days of CXB treatment did not produce significant changes in the enzymatic activities.
Microsomes of non-treated rats showed a production of acetaldehyde that was 3.5-fold higher than that of nitrites, which suggests that the predominant route for the DEN metabolism is deethylation, leading to the bioactivation of the carcinogen.
Microsomes obtained from CXB-treated rats showed a 3.6-fold increase in the rate of denitrosation of DEN, while there was no effect on DEN deethylation. This result indicates an induction of the detoxification pathway of the carcinogen. To confirm whether this effect resulted from induction of the enzymatic activity of CYP2B1/2 by CXB, we included SKF-525A in the assay, a CYP inhibitor that acts preferentially on this isoform. Inhibition of CYP2B1/2 resulted in a 98% reduction in nitrite production in the microsomes isolated from non-treated animals, and a 95% reduction in the microsomes of CXB-treated rats, suggesting that CYP2B1/2 was involved in the denitrosation of DEN under basal conditions (NT animals) and CXB-induced conditions. The deethylation rate in non-treated rat microsomes decreased 73%, and no statistically significant changes were observed in the rat microsomes treated with CXB. One possible explanation for this result is that CYP2E1 and 1A1 are minimally affected by SKF-525A and could be responsible for the acetaldehyde production under these conditions (Figure 3).
Figure 3.
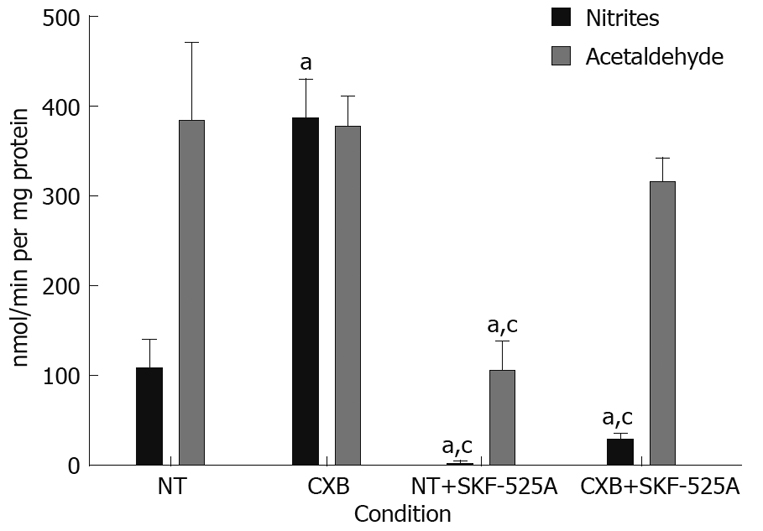
In vitro metabolism of DEN in non-treated and CXB-treated rat hepatic microsomes. The denitrosation rate was measured by the production of nitrites, and the deethylation rate was measured by the production of acetaldehyde in the presence or absence of SKF-525A. Values are presented as mean ± SD. aP < 0.05, vs NT group; cP < 0.05 vs CXB group. Bonferroni test, n = 4 for all groups.
DISCUSSION
CXB has shown to have anticancer effects in several experimental models, including the MRH model, where it showed a striking chemopreventive activity by inhibiting liver preneoplastic lesions in rats[8]. Although that study demonstrated a reduction in proliferation markers and in the nuclear translocation of NF-κB, the exact mechanism of action remains unclear[8].
Altered expression of CYP genes is a common feature in hepatic preneoplastic and neoplastic lesions induced by various carcinogens, including DEN[22]. Therefore, DEN metabolism via hepatic microsomal CYPs provides molecular targets for chemoprevention. This study demonstrates that the chemopreventive effect of CXB in the modified resistant hepatocyte model is mediated by changes in DEN metabolism via CYP regulation. CXB treatment for 8 d did not induce significant changes in enzymatic activities; however, when it was administered for 32 d or in combination with DEN, CXB strongly enhanced the enzymatic activity of CYP2B1/2. Moreover, CXB treatment increased the nitrite levels, which have been proposed to result from the DEN detoxification pathway[12,13]. This finding supports the explanation that the chemopreventive activity of CXB is carried out by reducing carcinogen-induced DNA damage, thus preventing the initiation of hepatocarcinogenesis. We propose that the effect of CXB is due to the preferential induction of CYP2B1/2. This hypothesis is reinforced by in vitro results, where the increase in DEN denitrosation elicited by CXB was inhibited by the addition of SKF-525A, an inhibitor of several CYP isoforms including 2B1/2B2, 3A1/2 and 2A, whereas CYP2E1 and 1A are less affected[23]. The deethylation rate was not affected by the inhibitor, suggesting that CYP2E1, and possibly CYP1A, could be the main isoforms involved in this pathway.
According to a previous report, the enzymatic activity of CYP2E1 increased with DEN treatment[24]. This is congruent with the participation of this isoform in DEN metabolism[10,24]. However, CXB did not have any effect on this increase, suggesting that there is no contribution of CYP2E1 to the chemoprotective effect of CXB. On the other hand, CXB reversed the effect of DEN on CYP1A1-specific EROD activity. A decrease in the CYP1A1 enzyme activity in preneoplastic lesions induced by DEN has been previously reported[25]. Induction of CYP1A1 has been related to chemoprevention[26]; thus, the induction of this isoform by CXB could explain its chemopreventive effect, but comparing the levels of enzymatic activity induced reveals that its participation is probably minor compared to CYP2B1/2. Additionally, CYP2A was not affected by CXB, and considering that it is partially affected by SKF-525A, this isoform could be involved in the deethylation reaction of DEN, although to a lesser extent than CYP2E1.
This is the first report that describes the effect of CXB on hepatic CYP regulation. Other chemoprotectors have been shown to act in a similar way. For example, among their multiple effects, diallyl sulfide, indole-3-carbinol, d-limonene and bicyclol induced the enzymatic activity of several CYP isoforms, including CYP2B1/2[21,26]. In particular, the effect of bicyclol on CYP2B1 was associated with an increase in the denitrosation rate of DEN[21]. In that case, bicyclol reduced the Km values for denitrosation below the values of deethylation, which may be attributed to the induction of specific CYPs. According to these results on DEN metabolism, the chemopreventive CXB effect in the MRH model could be similar to that of bicyclol[21], mediated mainly by the 2B1/2 isoform. Isoforms of the 1A, 2A, 2B and 2E CYP families share a broader overlap in substrate selectivity. In addition, a single enzyme can bind a variety of substrates, multiple substrates, and/or generate multiple products from a single substrate, which makes it difficult to discriminate between these possibilities in in vivo systems[27,28]. Further studies are required to clarify the mechanism by which CXB induces the denitrosation of DEN, and whether this is generated simply by the preferential induction of isoforms or by other effects.
In summary, the modulation of several hepatic CYPs by CXB modifies the bioactivation of DEN, favoring detoxification via denitrosation. This pathway may constitute an additional mechanism of action to explain the chemoprotective effects of CXB at the initiation stage in this hepatocarcinogenesis model.
COMMENTS
Background
Celecoxib, a non-steroidal antiinflammatory drug, is associated with a reduced incidence of several types of cancer, including hepatocellular carcinoma. Study of the mechanism of action has been possible by means of animal models. In the modified resistant hepatocyte model, celecoxib has shown a chemoprotector effect in the development of liver preneoplastic lesions; however, the action mechanism has not been defined completely.
Research frontiers
Diethylnitrosamine bioactivation is a crucial event in the initiation stage of the modified resistant hepatocyte model, a process dependent on hepatic cytochrome P450 (CYP). Therefore, modulation of liver CYP provides molecular targets for chemoprevention. This study demonstrates that the chemopreventive effect of celecoxib is mediated by changes in diethylnitrosamine metabolism via CYP regulation.
Innovations and breakthroughs
Recent investigations have demonstrated several mechanisms through which celecoxib exerts its chemoprotector effect. However, this is the first report that describes the capacity of celecoxib to modulate liver CYP expression and explains how the preferential induction of CYP2B1/2 activates the detoxification pathway by increasing nitrite formation. These effects represent an additional mechanism to elucidate the chemopreventive activity of celecoxib.
Applications
This study contributes to the understanding of the mode of action of celecoxib, which may represent a future strategy for therapeutic intervention in the treatment of patients with a high risk of suffering hepatocellular carcinoma.
Terminology
CYP is hepatic microsomal protein involved in the phase I metabolism. Celecoxib is a nonsteroidal anti-inflammatory drug that specifically inhibits cyclooxygenase-2. Diethylnitrosamine is a carcinogen initiator used in the modified resistant hepatocyte model.
Peer review
The authors examined the capability of celecoxib to modulate CYP as part of its chemopreventive mechanism in the modified resistant hepatocyte model. The results suggest that celecoxib favors the diethylnitrosamine detoxification and contribute to clarifying the chemopreventive mechanism in the chemical hepatocarcinogenesis of rat.
Acknowledgments
We thank Dr. Angelina Flores and Sonia Sánchez for their aid in the extraction of celecoxib, Dr. Víctor Pérez and Isabel Wens for their helpful assistance with HPLC-based determinations, and Patricia Vázquez, Evelia Arce and Sergio Hernández for their technical support. We also thank Maria Antonieta López, Rafael Leyva, Manuel Flores, Ricardo Gaxiola and UPEAL Chairman Dr. Jorge Fernández at UPEAL-CINVESTAV for the animal handle and care.
Supported by Consejo Nacional de Ciencia y Tecnología (Mexico), grant 39525-M, and scholarship 119303 (M.E.S.N.)
Peer reviewers: Jian Wu, Associate Professor of Medicine, Internal Medicine/Transplant Research Program, University of California, Davis Medical Center, 4635 2nd Ave. Suite 1001, Sacramento CA 95817, United States; Tetsuya Mine, MD, PhD, Professor, Chief, Director of Gastroenterological Center, Division of Gastroenterology and Hepatology, Department of Internal Medicine, University of Tokai School of Medicine, Bohseidai, Isehara, Kanagawa 259-1193, Japan
S- Editor Tian L L- Editor Logan S E- Editor Lin YP
References
- 1.Blum HE. Hepatocellular carcinoma: therapy and prevention. World J Gastroenterol. 2005;11:7391–7400. doi: 10.3748/wjg.v11.i47.7391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rüegg C, Zaric J, Stupp R. Non steroidal anti-inflammatory drugs and COX-2 inhibitors as anti-cancer therapeutics: hypes, hopes and reality. Ann Med. 2003;35:476–487. doi: 10.1080/07853890310017053. [DOI] [PubMed] [Google Scholar]
- 3.Kawamori T, Rao CV, Seibert K, Reddy BS. Chemopreventive activity of celecoxib, a specific cyclooxygenase-2 inhibitor, against colon carcinogenesis. Cancer Res. 1998;58:409–412. [PubMed] [Google Scholar]
- 4.Pentland AP, Schoggins JW, Scott GA, Khan KN, Han R. Reduction of UV-induced skin tumors in hairless mice by selective COX-2 inhibition. Carcinogenesis. 1999;20:1939–1944. doi: 10.1093/carcin/20.10.1939. [DOI] [PubMed] [Google Scholar]
- 5.Grubbs CJ, Lubet RA, Koki AT, Leahy KM, Masferrer JL, Steele VE, Kelloff GJ, Hill DL, Seibert K. Celecoxib inhibits N-butyl-N-(4-hydroxybutyl)-nitrosamine-induced urinary bladder cancers in male B6D2F1 mice and female Fischer-344 rats. Cancer Res. 2000;60:5599–5602. [PubMed] [Google Scholar]
- 6.Harris RE, Alshafie GA, Abou-Issa H, Seibert K. Chemoprevention of breast cancer in rats by celecoxib, a cyclooxygenase 2 inhibitor. Cancer Res. 2000;60:2101–2103. [PubMed] [Google Scholar]
- 7.Kern MA, Schubert D, Sahi D, Schöneweiss MM, Moll I, Haugg AM, Dienes HP, Breuhahn K, Schirmacher P. Proapoptotic and antiproliferative potential of selective cyclooxygenase-2 inhibitors in human liver tumor cells. Hepatology. 2002;36:885–894. doi: 10.1053/jhep.2002.36125. [DOI] [PubMed] [Google Scholar]
- 8.Márquez-Rosado L, Trejo-Solís MC, García-Cuéllar CM, Villa-Treviño S. Celecoxib, a cyclooxygenase-2 inhibitor, prevents induction of liver preneoplastic lesions in rats. J Hepatol. 2005;43:653–660. doi: 10.1016/j.jhep.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 9.Verna L, Whysner J, Williams GM. N-nitrosodiethylamine mechanistic data and risk assessment: bioactivation, DNA-adduct formation, mutagenicity, and tumor initiation. Pharmacol Ther. 1996;71:57–81. doi: 10.1016/0163-7258(96)00062-9. [DOI] [PubMed] [Google Scholar]
- 10.Bellec G, Goasduff T, Dreano Y, Menez JF, Berthou F. Effect of the length of alkyl chain on the cytochrome P450 dependent metabolism of N-diakylnitrosamines. Cancer Lett. 1996;100:115–123. doi: 10.1016/0304-3835(95)04078-1. [DOI] [PubMed] [Google Scholar]
- 11.Yamazaki H, Inui Y, Yun CH, Guengerich FP, Shimada T. Cytochrome P450 2E1 and 2A6 enzymes as major catalysts for metabolic activation of N-nitrosodialkylamines and tobacco-related nitrosamines in human liver microsomes. Carcinogenesis. 1992;13:1789–1794. doi: 10.1093/carcin/13.10.1789. [DOI] [PubMed] [Google Scholar]
- 12.Janzowski C, Pool BL, Preussmann R, Eisenbrand G. Fluoro-substituted N-nitrosamines. 2. Metabolism of N-nitrosodiethylamine and of fluorinated analogs in liver microsomal fractions. Carcinogenesis. 1982;3:155–159. doi: 10.1093/carcin/3.2.155. [DOI] [PubMed] [Google Scholar]
- 13.Appel KE, Rühl CS, Hildebrandt AG. Metabolic inactivation of N-nitrosamines by cytochrome P-450 in vitro and in vivo. IARC Sci Publ. 1984;3:443–451. [PubMed] [Google Scholar]
- 14.Wade D, Yang CS, Metral CJ, Roman JM, Hrabie JA, Riggs CW, Anjo T, Keefer LK, Mico BA. Deuterium isotope effect on denitrosation and demethylation of N-nitrosodimethylamine by rat liver microsomes. Cancer Res. 1987;47:3373–3377. [PubMed] [Google Scholar]
- 15.Mayer RT, Netter KJ, Heubel F, Hahnemann B, Buchheister A, Mayer GK, Burke MD. 7-Alkoxyquinolines: new fluorescent substrates for cytochrome P450 monooxygenases. Biochem Pharmacol. 1990;40:1645–1655. doi: 10.1016/0006-2952(90)90467-y. [DOI] [PubMed] [Google Scholar]
- 16.Burke MD, Thompson S, Elcombe CR, Halpert J, Haaparanta T, Mayer RT. Ethoxy-, pentoxy- and benzyloxyphenoxazones and homologues: a series of substrates to distinguish between different induced cytochromes P-450. Biochem Pharmacol. 1985;34:3337–3345. doi: 10.1016/0006-2952(85)90355-7. [DOI] [PubMed] [Google Scholar]
- 17.Lubet RA, Mayer RT, Cameron JW, Nims RW, Burke MD, Wolff T, Guengerich FP. Dealkylation of pentoxyresorufin: a rapid and sensitive assay for measuring induction of cytochrome(s) P-450 by phenobarbital and other xenobiotics in the rat. Arch Biochem Biophys. 1985;238:43–48. doi: 10.1016/0003-9861(85)90138-9. [DOI] [PubMed] [Google Scholar]
- 18.Reinke LA, Moyer MJ. p-Nitrophenol hydroxylation. A microsomal oxidation which is highly inducible by ethanol. Drug Metab Dispos. 1985;13:548–552. [PubMed] [Google Scholar]
- 19.Sierra-Santoyo A, Hernández M, Albores A, Cebrián ME. DDT increases hepatic testosterone metabolism in rats. Arch Toxicol. 2005;79:7–12. doi: 10.1007/s00204-004-0603-y. [DOI] [PubMed] [Google Scholar]
- 20.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 21.Zhu B, Liu GT, Wu RS, Strada SJ. Chemoprevention of bicyclol against hepatic preneoplastic lesions. Cancer Biol Ther. 2006;5:1665–1673. doi: 10.4161/cbt.5.12.3377. [DOI] [PubMed] [Google Scholar]
- 22.Roomi MW, Ho RK, Sarma DS, Farber E. A common biochemical pattern in preneoplastic hepatocyte nodules generated in four different models in the rat. Cancer Res. 1985;45:564–571. [PubMed] [Google Scholar]
- 23.Ono S, Hatanaka T, Hotta H, Satoh T, Gonzalez FJ, Tsutsui M. Specificity of substrate and inhibitor probes for cytochrome P450s: evaluation of in vitro metabolism using cDNA-expressed human P450s and human liver microsomes. Xenobiotica. 1996;26:681–693. doi: 10.3109/00498259609046742. [DOI] [PubMed] [Google Scholar]
- 24.Liu LL, Gong LK, Qi XM, Cai Y, Wang H, Wu XF, Xiao Y, Ren J. Altered expression of cytochrome P450 and possible correlation with preneoplastic changes in early stage of rat hepatocarcinogenesis. Acta Pharmacol Sin. 2005;26:737–744. doi: 10.1111/j.1745-7254.2005.00737.x. [DOI] [PubMed] [Google Scholar]
- 25.Buchmann A, Schwarz M, Schmitt R, Wolf CR, Oesch F, Kunz W. Development of cytochrome P-450-altered preneoplastic and neoplastic lesions during nitrosamine-induced hepatocarcinogenesis in the rat. Cancer Res. 1987;47:2911–2918. [PubMed] [Google Scholar]
- 26.Guengerich FP. Influence of nutrients and other dietary materials on cytochrome P-450 enzymes. Am J Clin Nutr. 1995;61:651S–658S. doi: 10.1093/ajcn/61.3.651S. [DOI] [PubMed] [Google Scholar]
- 27.Harrelson JP, Henne KR, Alonso DO, Nelson SD. A comparison of substrate dynamics in human CYP2E1 and CYP2A6. Biochem Biophys Res Commun. 2007;352:843–849. doi: 10.1016/j.bbrc.2006.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Atkins WM. Non-Michaelis-Menten kinetics in cytochrome P450-catalyzed reactions. Annu Rev Pharmacol Toxicol. 2005;45:291–310. doi: 10.1146/annurev.pharmtox.45.120403.100004. [DOI] [PubMed] [Google Scholar]