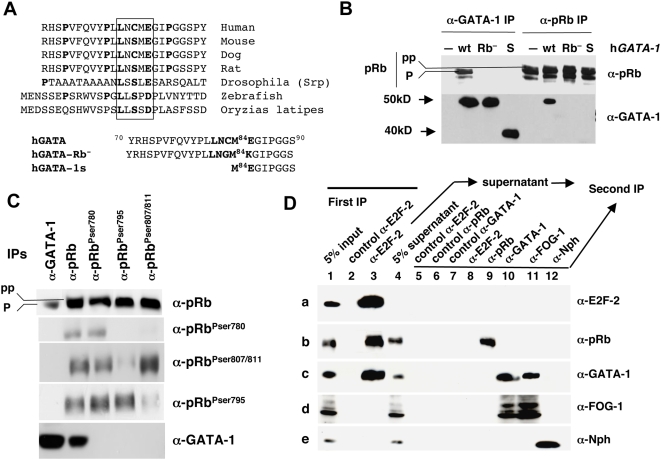
Figure 1. The GATA-1 LXCXE motif mediates the GATA-1/pRb interaction.
(A) Top, protein sequence alignment of GATA-1 orthologs. The consensus pRb association motif, LXCXE, is boxed. Bottom, protein sequence alignment of GATA-1 mutants used in Figure 2B. (B) Co-IP assays of nuclear lysates from retrovirally transduced NIH-3T3 cells expressing wt hGATA-1, hGATA-1 bearing two amino acid substitutions that disrupt the LNCME motif (Rb−), or hGATA-1 lacking 83 NH2 amino acids (S). Corresponding amino acid sequences are indicated at the bottom of Figure 1A. The initial IP step was performed with either anti(α)-GATA-1 (C-20; Santa Cruz Biotechnology), which recognizes both wt and S forms of GATA-1, or anti(α)-pRb (BD Pharmingen) Abs. The immunoprecipitates were subsequently resolved by western blot analysis with either one of the same Abs, i.e., anti(α)-pRb or anti(α)-GATA-1. Hypo (“p”) and hyperphosphorylated (“pp”) pRb forms are indicated. An identical experiment, demonstrating that signals were not immunoglobulin artifacts (IgG isotype species-specific controls for all the Abs used), is shown in Figure S1B. (C) Co-IPs of nuclear extracts from the erythroid cell line UT-7 were performed with either (α)-GATA-1 (C20; Santa Cruz Biotechnology) or (α)-pRb (BD Pharmingen) Abs. Four different anti-pRb Abs were used with different specificities: α-pRb (BD Pharmingen) recognizes all forms of pRb regardless of their phosphorylation status; the other three Abs recognize pRb phosphorylated on either Ser780, Ser795, or Ser807/811 (Cell Signaling Technology). The precipitated proteins were subsequently analyzed by western blot using the same pRb Abs or an α-GATA-1 Ab (N1; Santa Cruz Biotechnology). (D) A first IP of nuclear extracts from purified CD71+ (>90 TER119+) late-stage erythroid cells from E12.5 mouse fetal liver was performed with an α-E2F-2 Ab. The first IP immunoprecipitate was analyzed in lane 3, with its IgG species-specific isotype control (lane 2). The E2F-2 immunodepleted supernatant was then submitted to secondary IPs with either α-E2F-2 (lane 8), α-pRb (lane 9), α-GATA-1 (lane 10), α-FOG-1 (lane 11), or the negative control α-Nucleophosmin (Nph) (lane 12) Abs. The relevant IgG species-specific isotype controls are shown in lanes 5, 6, and 7 (see experimental procedure in Figure S2E). Initial 5% input and 5% supernatant controls were analyzed in lanes 1 and 4, respectively. Primary (α-E2F-2) IP, secondary IPs, and the aforementioned controls were subsequently resolved by western blot analysis with the indicated Abs (rows a to e).