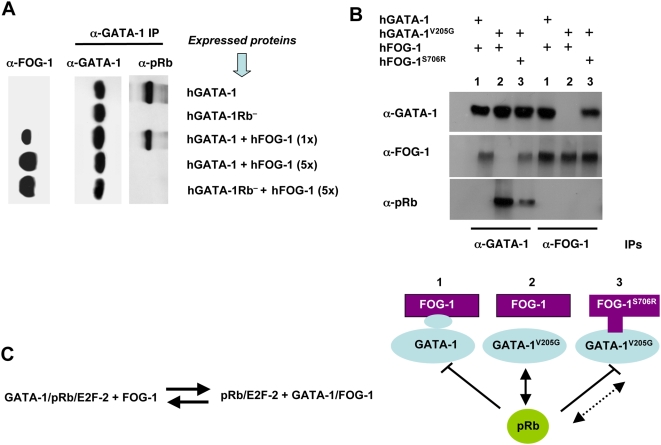
Figure 2. FOG-1 interferes with GATA-1/pRb association.
(A) Co-IPs, by means of an anti(α)-GATA-1 Ab, of nuclear extracts from retrovirally transduced NIH-3T3 cells expressing hGATA-1 or hGATA-1Rb− and from the same cells after subsequent transient transfection with increasing amounts (1- to 5-fold, with 1-fold = 0.1 µg) of a hFOG-1 expression plasmid. We verified that >90% cells were successfully transduced by each of the retroviral vectors on the basis of coexpression of eGFP from an internal ribosome entry site (IRES). Immunoprecipitates were then resolved by western blot analysis with either anti(α)-GATA-1 or anti(α)-pRb antibodies, under identical experimental conditions as for Figure 1B and S1. The amount of hFOG-1 expressed after transient transfection was assessed in parallel on total nuclear extracts with an anti(α)-FOG-1 Ab (left column). (B) Nuclear extracts were made from retrovirally transduced NIH-3T3 cells expressing hGATA-1, or hGATA-1V205G, which cannot interact with hFOG-1, or 3 d after transient transfection with a plasmid expressing hFOG-1 or an identical amount of an expression plasmid encoding hFOG-1S706R, which can interact with hGATA-1V205G. We verified that >90% cells were successfully transduced by each of the retroviral vectors on the basis of coexpression of eGFP from an IRES. Co-IP assays were performed with these nuclear extracts by means of α-GATA-1 and α-FOG-1 Abs, followed by western blot analysis with either α-GATA-1, α-FOG-1, or α-pRb Abs. A schematic representation of the various combinations of FOG and GATA variants expressed (lines 1 to 3) is indicated at the bottom of the figure. (C) A proposed protein equilibrium model.