Abstract
Background and Purpose
Electrocardiographic (ECG) abnormalities are common after subarachnoid hemorrhage (SAH) but their significance remains uncertain. The aim of this study was to determine whether any specific ECG abnormalities are independently associated with adverse neurological outcomes.
Methods
This was a sub-study of the Intraoperative Hypothermia Aneurysm Surgery Trial, which was designed to determine whether intraoperative hypothermia would improve neurological outcome in SAH patients undergoing aneurysm surgery. The outcome was the 3 month Glasgow Outcome Score (GOS), treated as both a categorical measure [GOS 1 (good outcome) to 5 (death)] and dichotomously (mortality/GOS 5 vs GOS 1−4.). The predictor variables were pre-operative ECG characteristics, including heart rate (HR), corrected QT interval, and ST and T wave abnormalities. Univariate logistic regression was performed to screen for significant ECG variables, which were then tested for associations with the outcome by multivariate logistic regression, adjusting for clinical covariates.
Results
The study included 588 patients, of which 31 (5%) died. There was a significant, non-linear association between heart rate and mortality such that lowest quartile (≤60 bpm, odds ratio [OR] 6.5, P=0.027) and highest quartile (>80 bpm, OR 8.8, P=0.006) were associated with higher risk. There was also a significant association between non-specific ST and T wave abnormalities (NSSTTWA) and mortality (OR 3.1, P=0.031).
Conclusions
Bradycardia, relative tachycardia, and NSSTTWA are strongly and independently associated with 3 month mortality after SAH. Further research should be performed to determine whether or not there is a causal relationship between cardiac dysfunction and neurological outcome after SAH.
Keywords: Subarachnoid hemorrhage, electrocardiography, bradycardia
Introduction
Cardiac injury and dysfunction after SAH is a well-recognized phenomenon. In 1954, Burch described “cerebral T wave” electrocardiographic (ECG) abnormalities in patients with stroke and noted that the findings were most marked in SAH patients.1 Since then, diverse ECG changes have been described and occur in 25 to 90% of SAH patients.2-4 Prior studies have described associations between ECG changes and adverse neurological outcomes after SAH but these studies had limited power and limited ability to statistically adjust for known predictors of poor outcome after SAH.3, 5-7 Because patients with more severe SAH are more likely to develop both cardiac abnormalities3, 7 and poor neurological outcome,8 it has been unclear whether ECG changes are, in fact, independently associated with outcome.
The aim of this study was to test the hypothesis that ECG abnormalities occurring in preoperative World Federation of Neurological Surgeons (WFNS) class I − III SAH patients prior to surgical aneurysm clipping are independently associated with poor neurologic outcome after adjustment for relevant covariates.
Materials and Methods
This was a sub-study of the Intraoperative Hypothermia for Aneurysm Surgery Trial (IHAST). IHAST was a multi-center, prospective, randomized, partially-blinded clinical trial designed to determine whether mild intraoperative systemic hypothermia (33°C) would result in improved neurological outcome in patients undergoing surgery to treat ruptured intracranial aneurysms as compared with intraoperative normothermia. Details of trial design, patient eligibility, protocols, and outcomes have been published previously.9 In brief, adults with subarachnoid hemorrhage (SAH) and a radiographically-confirmed intracranial aneurysm who were scheduled to undergo surgical treatment of the aneurysm within 14 days of SAH were eligible to participate. Other major criteria included a preoperative WFNS class of I, II, or III,10 and the absence of endotracheal intubation at the time of study enrollment. IHAST protocols were approved by the Human Subjects’ Committees at each participating center (n=30) and written informed consent was obtained from either patients or their families. There were 1001 patients enrolled into IHAST. The study's main finding was that intraoperative hypothermia did not affect neurological outcome.9
IHAST data collection included patient demographics and pre-SAH medical history. Pre-SAH hypertension was defined as being definitely or probably present. Pre-SAH coronary disease was defined as a history of one or more of the following: myocardial infarction, angina, positive stress test, or coronary revascularization. Information regarding the characteristics of the ruptured aneurysm (location, angiographic diameter), and its immediate effect (extent of subarachnoid blood [Fisher Scale],11 preoperative WFNS class,10 and National Institutes of Health Stroke Scale [NIHSS])12 were recorded prior to surgery. Posterior circulation aneurysms included vertebrobasilar and posterior inferior cerebellar arteries. Anterior circulation aneurysms included carotid or ophthalmic, posterior communicating, anterior choroidal, carotid bifurcation, carotid (other), middle cerebral, anterior communication, and anterior cerebral (other) arteries.
A final follow-up examination was conducted approximately 3 months after surgery by certified examiners who were unaware of intraoperative temperature management. The primary outcome measure, the 5-point modified Glasgow Outcome Score (GOS),13 was obtained in 1000 patients (1 = good recovery, 2 = moderate disability, 3 = severe disability, 4 = vegetative state, 5 = death). All deaths were reviewed and adjudicated as to primary cause by a designated physician blinded to temperature assignment and summarized using ICD-10 codes.
In IHAST, the occurrence of a large number of defined clinical events was prospectively documented. Examples of cardiovascular and neurologic outcomes assessed on a daily basis included: congestive heart failure or pulmonary edema (by clinical assessment or chest X-ray), hypotension (undesired fall in mean arterial pressure ≤ 60 mmHg for ≥15 consecutive minutes (or longer), vasopressor/inotrope use to support cardiovascular function (continuously administered for ≥15 consecutive minutes), ventricular arrhythmia (≥3 consecutive wide complex (≥120 msec) beats at a rate ≥100 beats/minute), and cerebral infarction (neurologic deficits or the presence of abnormalities on brain images indicating loss of viability).
ECG Sub-Study Protocol
The ECG sub-study was designed and implemented in 2001, during ongoing enrollment of IHAST. IHAST protocols did not require a preoperative electrocardiogram (ECG), although a 12-lead ECG was part of the routine preoperative assessment of the majority of patients. Therefore, IHAST centers were asked to include any preoperative ECGs that were obtained as part of routine care. To increase the likelihood of an association between ECG abnormalities and SAH, an a priori decision was made to include only patients undergoing surgery within 4 days (96 h) of SAH. Exclusion criteria for this sub-study were a pre-SAH history of arrhythmias and the presence of a temporary or permanent pacemaker.
When patients had multiple preoperative ECG's, the ECG obtained closest to the time of SAH was used for analysis. Patient identifiers were removed from the ECGs which were then analyzed by cardiologists (JZ, NB) at a single site (University of California, San Francisco), who were blinded to temperature group assignments, clinical characteristics, and outcomes. The following measurements were made for each ECG: heart rate (ventricular rate), primary rhythm, QT interval (absolute), and heart-rate corrected QT interval (QTc, Bazett's formula). Each ECG was also characterized as to the presence of any of seven pre-defined morphological abnormalities (Table 1). To be considered abnormal, a morphological abnormality was required to be present in at least two leads within at least one ECG distribution (inferior [leads II, III, aVF], lateral [I, aVL], or anterior [V1-V6]).
Table 1.
Definitions of Morphological ECG Abnormalities
| ECG Abnormality | Diagnostic Criteria |
|---|---|
| Abnormal Q or QS wave | ≥ 30 ms or a pathological R wave in V1-V2 |
| ST Elevation | ST elevation ≥0.1 mV |
| ST Depression | ST depression ≥0.1 mV, 80 msec post-J point |
| Peaked upright T wave | Prominent peaked T wave |
| T wave inversions | Pathologic T wave inversion |
| Giant T wave inversions | T wave inversions > 10 mV in depth |
| Non-specific STTWA | ST or T wave abnormalities not meeting above criteria |
Statistical Methods
In order to assess the generalizability of the results, we compared the clinical characteristics of the ECG sub-study population with those IHAST patients who had surgery ≤4 days after SAH but who did not have a pre-operative ECG. We also compared IHAST subjects with surgery ≤4 days versus >5 days after SAH. These comparisons were made using Kruskal-Wallis and Chi-Square tests, according to the nature of each variable.
The primary aim was to test for associations between ECG variables and two different 3-month outcome measures: 1) GOS as a categorical outcome (1−5) and 2) mortality (GOS 5 vs.GOS 1−4). Pre-study assumptions included a 10% overall study mortality, a 15% incidence of preoperative ECG abnormalities, and a 2.4-fold increased risk of all-cause mortality in patients with preoperative ST/T wave abnormalities.3 Based on these pre-study assumptions, and an 80% power to detect a difference in all-cause mortality between groups (with ST/T abnormalities vs. without ST/T abnormalities), the investigators determined a sample size of 573 ECGs would be required to obtain a statistically significant result (alpha = 0.05). The study enrollment of 588 patients met this criterion.
For the primary study analysis, the step one models quantified the univariate associations between the ECG variables and the two outcome measures (GOS 1−5, mortality) using Fisher's exact (FE) test, analysis of variance (AOV), and t-tests (TT), as determined by the nature of each variable. Each of the seven morphologic ECG abnormalities was treated as a dichotomous variable (present or absent). The rate corrected QT interval (QTC) was used as a continuous variable. Because the relationship between heart rate and the outcomes was observed to be non-linear by visual inspection and logistic modeling, the ventricular rate was divided into four quartiles (≤60 bpm, 61−70, 71−80, 81−138) and treated as a categorical predictor variable.
The step two models employed multivariate stepwise selection methods to determine which ECG variables were independent of one another in their associations with the two outcome measures. All ECG variables with P values ≤ 0.30 were initially included in the models, but P values < 0.10 were required for variables to remain in the models.
The step three models employed multivariate stepwise selection methods to determine which ECG variables were independently associated with outcomes after inclusion of relevant clinical covariates. These later factors were determined a priori and were selected to characterize patient demographics (age, gender), aneurysm characteristics (size, location), severity of SAH (Fisher scale), and immediate neurologic sequelae (preoperative WFNS class, NIH Stroke Scale [treated as a continuous variable]). Also, because IHAST patients were randomized to one of two intraoperative temperatures (normothermia vs. hypothermia), this assignment was also included in this analysis. All of the pre-selected clinical covariates and all ECG variables were tested for inclusion in the final model, with a P value ≤ 0.30 for initial inclusion and P<0.10 for final retention.
There was complete data for all of the clinical covariates with two exceptions. Thirty-four patients had an incomplete NIHSS exam, almost always due to a leg that could not be moved after angiography. For these 34 patients, an imputed NIHSS score was calculated based on the NIHSS items that were present, normalized to a maximum score of 42. Two patients had missing values for aneurysm angiographic diameter. An imputed value for aneurysm diameter was obtained by taking the median value of aneurysm diameter of other patients in the ECG study population who were matched for age (±5 years), gender, aneurysm location, preoperative WFNS, NIHSS, and Fisher scores.
The study also included a secondary analysis, designed to explore for possible mechanisms underlying associations between the ECG variables and outcomes. For this analysis, additional multivariate models that included clinical covariates were generated using the significant ECG predictors from the primary analysis (HR, QTc, nonspecific ST/T waves) and the pre-defined cardiovascular and neurological hospital outcomes (see methods above).
All statistical analyses were performed on SAS 9.1.3 Service Pack XP_PRO Platform. Logistic regression models reported odds ratios (OR), 95% confidence intervals (CI), and a P value < 0.05 was considered statistically significant.
Results
There were 778 patients who underwent surgery within 4 days of their SAH. The clinical characteristics and outcomes are shown in Table 2 and these did not differ significantly among patients who did (n=588, 76%) and did not (n=190, 24%) have a preoperative ECGs. The 222 IHAST subjects who had surgery ≥ 5 days after SAH had lower preoperative WFNS, Fisher, and NIHSS scores in comparison to those subjects having surgery within 4 days. No patients were excluded from the study on the basis on a prior history of arrhythmia or a pacemaker.
Table 2.
Population Characteristics and Primary Outcomes
| Characteristic |
Surgery ≤ 4 days after SAH (n=778) |
|
Surgery ≥5 days after SAH (n=222) |
|
Total (n=1000) |
|
|---|---|---|---|---|---|---|
| |
Preoperative ECG (n=588) |
No Preoperative ECG (n=190) |
P value* |
|
P value** |
|
|
Age (years, mean±SD) |
52±12 |
51±14 |
0.18† |
52±12 |
0.60† |
52±13 |
| Sex | 0.15†† | 0.82†† | ||||
| -Male | 210 (36%) | 57 (30%) | 78 (35%) | 345 (35%) | ||
| -Female |
378 (64%) |
133 (70%) |
|
144 (65%) |
|
655 (66%) |
| WFNS Class | 0.92†† | <0.001†† | ||||
| -I | 369 (63%) | 119 (63%) | 172 (78%) | 660 (66%) | ||
| -II | 187 (32%) | 62 (33%) | 40 (18%) | 289 (29%) | ||
| -III |
32 (5%) |
9 (5%) |
|
10 (5%) |
|
51 (5%) |
| Fisher Class | 0.45†† | <0.001†† | ||||
| -I | 21 (4%) | 4 (2%) | 29 (13%) | 54 (5%) | ||
| -II | 202 (34%) | 61 (32%) | 79 (36%) | 342 (34%) | ||
| -III | 299 (51%) | 97 (51%) | 78 (47%) | 474 (47%) | ||
| -IV |
66 (11%) |
28 (15%) |
|
36 (16%) |
|
130 (13%) |
| NIHSS Score | 0.20†† | 0.002†† | ||||
| -Incomplete exam | 34 (6%) | 20 (11%) | 2 (1%) | 56 (6%) | ||
| -No Deficit (0) | 305 (52%) | 95 (50%) | 135 (61%) | 535 (54%) | ||
| -Mild (1−7) | 232 (40%) | 68 (36%) | 75 (34%) | 375 (38%) | ||
| -Moderate (8−14) | 14 (2%) | 5 (3%) | 7 (3%) | 26 (3%) | ||
| -Severe (≥15) |
3 (1%) |
2 (1%) |
|
3 (1%) |
|
8 (1%) |
| Aneurysm Location | 0.66†† | 0.010†† | ||||
| -Anterior | 547 (93%) | 174 (92%) | 194 (87%) | 915 (92%) | ||
| -Posterior |
41 (7%) |
15 (8%) |
|
28 (13%) |
|
84 (8%) |
| Aneurysm Size | 0.42†† | 0.94†† | ||||
| −1−11 mm | 471 (80%) | 155 (82%) | 178 (80%) | 808 (81%) | ||
| −12−24 mm | 101 (17%) | 27 (14%) | 36 (16%) | 164 (16%) | ||
| -≥25 mm | 14 (2%) | 7 (4%) | 7 (3%) | 28 (3%) | ||
| -Missing value |
2 (<1%) |
1 (<1%) |
|
1 (<1%) |
|
|
| History of Hypertension |
238 (41%) |
75 (40%) |
0.81†† |
85 (38%) |
0.60†† |
398 (40%) |
| History of CAD |
33 (6%) |
10 (5%) |
0.86†† |
10 (5%) |
0.549†† |
53 (5%) |
| SAH to Surgery (days) (mean±SD) |
1.9±1.2 |
1.8±1.1 |
0.26† |
7.8±2.7 |
<0.001†† |
3.2±2.9 |
| 3-Month GOS Score | 0.12†† | 0.743 | ||||
| −1 (Good Outcome) | 378 (64%) | 122 (64%) | 143 (64%) | 643 (64%) | ||
| −2 (Mild Disability) | 128 (22%) | 39 (21%) | 46 (21%) | 213 (21%) | ||
| −3 (Moderate Disability) | 50 (9%) | 10 (5%) | 22 (10%) | 82 (8%) | ||
| −4 (Vegetative) | 1 (0%) | 0 (0%) | 0 (0%) | 1 (<1%) | ||
| −5 (Death) | 31 (5%) | 19 (10%) | 11 (5%) | 61 (6%) | ||
SAH = Subarachnoid hemorrhage. ECG = electrocardiogram. WFNS = World Federation of Neurological Surgeons. NIHSS = National Institutes of Health Stroke Scale. CAD = coronary artery disease. GOS = Glasgow Outcome Score.
P value for differences between the ECG and non-ECG populations
P value for difference between subjects with surgery ≤4 days versus ≥5 days after SAH
Kruskal-Wallis test
Pearson's Chi-Square test
In the ECG subpopulation, 378 (64%) had a good three-month outcome (GOS 1) and 31 (5%) died. In 23 patients (74%), neurological injury (cerebral infarction, cerebral edema, intracranial hypertension, hydrocephalus, etc.) was the primary cause of death. Of these 23 patients, 2 had fatal anoxic brain injury following resuscitation from cardiac arrest. Of the remaining 8 patients, 2 deaths were due to sepsis and its sequelae, and 6 deaths were primarily on a respiratory basis (including two patients with fatal pulmonary emboli). Of the 6 patients dying primarily on a respiratory basis, 3 had co-existing neurologic injuries. Therefore, neurological injuries were the primary or contributing cause of death in 26 (84%) of the patients. Bradycardia and hypotension were coded as minor contributors to death in 1 patient each.
Table 3 summarizes the study's ECG findings. At least one morphological ECG abnormality was present in 468 patients (80%) and a total of 907 different abnormalities were present. The most common abnormality was non-specific ST-T wave changes (NSSTTWA), followed by ST elevation, T wave inversion, and ST depression. ECG abnormalities were most commonly observed in the anterior leads. There were no significant associations between the number or type of observed ECG abnormalities and either the preoperative WFNS or Fisher class. Compared to a published study of pre-operative ECGs in patients undergoing any type of non-cardiac surgery (with a mean age of 60), the SAH study patients were more likely to have an abnormal ECG (80% vs 25%) and ST depression (7% vs 3%) but less likely to have Q waves (6% vs 10%).14
Table 3.
Preoperative ECG Characteristics
| Variable |
Value |
|||
|---|---|---|---|---|
| Fundamental Rhythm | ||||
| -Normal Sinus | 421 (72%) | |||
| -Sinus Bradycardia (<60) | 124 (21%) | |||
| -Sinus Tachycardia (≥100) | 27 (5%) | |||
| -Atrial Fibrillation | 6 (1%) | |||
| -Other Supraventricular Rhythm |
10 (2%) |
|||
| Ventricular Rate | ||||
| -Mean ±SD | 72 ±15 | |||
| -Median | 70 | |||
| -Quartile ranges |
36−60, 61−70, 71−80, 81−138 |
|||
| QT interval-absolute (msec) | ||||
| -Mean ±SD | 402 ±49 | |||
| -Median | 397 | |||
| -Range |
264−700 |
|||
| Corrected QT interval (QTc) (msec) | ||||
| -Mean ±SD | 433 ±39 | |||
| -Median | 427 | |||
| -Range | 350−658 | |||
| -QTc > 440, N (%) |
205 (35%) |
|||
| ECG abnormalities in patients who had at least one abnormality (N=468): |
Distribution |
|||
| |
Inferior |
Lateral |
Anterior |
Total |
| -Non-specific ST-T wave abnormalities | 214 | 168 | 179 | 561 (62%) |
| -ST elevation | 2 | 3 | 113 | 118 (13%) |
| -T wave inversion | 16 | 17 | 50 | 83 (9%) |
| -ST depression | 26 | 0 | 36 | 62 (7%) |
| -Abnormal Q or QS | 25 | 7 | 18 | 50 (6%) |
| -Peaked upright T wave | 5 | 0 | 24 | 29 (3%) |
| -Giant T wave inversion | 0 | 0 | 4 | 4 (<1%) |
| TOTAL (% of total) | 288 (32%) | 195 (21%) | 424 (47%) | 907 (100%) |
For the primary analysis, the step one models showed that for GOS (1−5) ST depression had a significant univariate association with outcome (FE P = 0.026), and both NSSTTWA (FE P = 0.085) and QTc (AOV P = 0.051) had non-significant associations. Four ECG variables had significant univariate associations with mortality (GOS5): NSSTTWA (FE P = 0.020), ST depression (FE P = 0.047), heart rate (FE P = 0.019), and QTc (TT P = 0.038).
The step two models, designed to test the independence of the ECG variables from each other (but without inclusion of clinical covariates), showed that, for GOS (1−5), ST Depression (OR 2.0, CI 1.2−3.5, P = 0.011) and QTc (OR 1.004 per 1 msec increase, CI 1.000−1.009, P = 0.043) were significantly associated outcome, and Q/QS waves had a non-significant association (OR 1.7, CI 0.9−3.0, P = 0.076). Both NSSTTWA (OR 3.0, CI 1.1−8.1, P=0.026) and QTc (OR 1.009 per 1 msec increase, CI 1.002−1.016, P=0.018) were significantly associated with mortality.
The results for the step three GOS (1−5) model, that included clinical covariates, are shown in Table 4. Age, preoperative WFNS, posterior aneurysm location, and aneurysm size were significant and independent predictors of GOS score. In this model, there was only a non-significant association between QTc and GOS score.
Table 4.
Multivariate Predictors of GOS Score (1−5)
| Variable | Odds Ratio*(95% CI) | p-value |
|---|---|---|
| Age (per 1 year↑) | 1.025 (1.011−1.040) | 0.001 |
| †WFNS: II vs. I | 2.4 (1.7 − 3.5) | <0.001 |
| WFNS: III vs. I | 4.0 (2.0 − 7.9) | <0.001 |
| Posterior aneurysm (vs. anterior) | 2.5 (1.3 − 4.5) | 0.004 |
| Aneurysm size (per 1 mm ↑) | 1.04 (1.01 − 1.07) | 0.014 |
| QTc (per 1 msec ↑) | 1.004 (1.000 − 1.008) | 0.071 |
Odds ratio indicates relative odds of a single category increase (worsening) in GOS score.
Omnibus P value for WFNS <0.001 with two degrees of freedom.
Model c-statistic = 0.671.
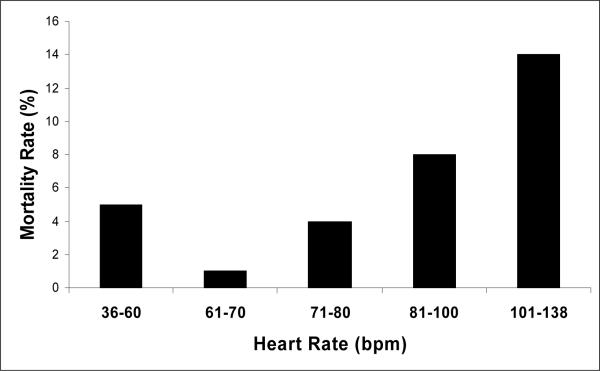
The results for the step three mortality model, that included clinical covariates, are shown in Table 5. Age and preoperative WFNS grade were the only clinical variables that remained in this model. Heart rate had a non-linear relationship with mortality such that 2nd quartile rates (61−70 bpm) were associated with lowest risk, and both the lowest and highest heart rate quartiles were associated with increased mortality (Figure 1). There was also a significant association between NSSTTWA and mortality; QTc had a non-significant association.
Table 5.
Multivariate Predictors of Mortality (GOS5)
| Variable | Odds Ratio*(95% CI) | p-value |
|---|---|---|
| Age (per 1 year ↑) | 1.04 (1.01 −1.07) | 0.023 |
| †WFNS: II vs. I | 2.4 (1.0 − 5.5) | 0.048 |
| WFNS: III vs. I | 10.9 (3.5 − 33.4) | <0.001 |
| NSSTTWA | 3.1 (1.1 − 8.6) | 0.031 |
| QTc (per 1 msec ↑) | 1.007 (0.999 − 1.015) | 0.095 |
| ‡HR ≤60 vs. 61−70 | 6.1 (1.2 − 30.2) | 0.028 |
| HR 71−80 vs. 61−70 | 3.9 (0.8 − 20.0) | 0.104 |
| HR 81−138 vs. 61−70 | 8.8 (1.8 − 42.3) | 0.006 |
Odds ratio indicates relative odds of mortality.
Omnibus P value for WFNS <0.001 with two degrees of freedom.
HR = heart rate in beats per minute
Omnibus P value for HR =0.040 with three degrees of freedom.
Model c-statistic = 0.798
Figure 1.
The Y axis indicates the unadjusted 3 month mortality rate. The first three bars from the left on the X axis indicate the lower three quartiles quartiles of heart rate on the pre-operative ECG. The top quartile is divided in the figure into 81−100 bpm (N=127)and 101−138 bpm (N=22). The combined mortality rate for the top quartile was 9%. A non-linear relationship is demonstrated as the lowest and highest heart rate quartiles are associated with the highest mortality rates.
Secondary analyses explored associations between the ECG predictors (heart rate, QTc, and NSSTTWA) and cardiovascular and neurological hospital outcomes. There was a significant association between QTc and postoperative hypotension (OR 1.011 per 1 msec increase, CI 1.00−1.02, P=0.048), which occurred in 16 (3%) of the ECG study subjects. There was also an association between top quartile heart rates and treatment with vasopressors or inotropes for cardiovascular indications (HR >80 vs. 61−70, OR 2.5, CI 1.3−5.0, P=0.008). Finally, the presence of NSSTTWA was associated with the use of vasopressors for cardiovascular indications (OR 2.2, CI 1.2−4.1, P=0.010 and with pulmonary edema (OR 1.9, CI 1.0−3.5, P=0.043).
Discussion
The primary result of this study is that pre-operative bradycardia (HR≤60 bpm), relative tachycardia (HR>80), and nonspecific ST segment and T wave abnormalities (NSSTTWA) were associated with increased mortality in SAH patients treated with surgical aneurysm clipping. These associations were independent of clinical covariates known to predict outcome after SAH, including age, aneurysm location, Fisher scale, preoperative WFNS, and NIHSS. In fact, the odds ratios for these ECG predictors were similar in magnitude to those observed for age and WFNS. It is notable that aneurysm size and location, Fisher scale and NIHSS were not significantly associated with mortality, suggesting that the ECG findings may actually have greater prognostic value than many of the more standard clinical measures. There were also non-significant associations between increasing QTc and both the categorical GOS and mortality outcomes.
These findings are novel compared to prior reports3, 5-7 and are likely attributable to the unique design of this study. Specifically, this is the largest study of this type to date and, in contrast to prior studies, the ECG data were collected prospectively and characterized based on pre-defined criteria. In addition, the outcomes and adverse events were assessed prospectively. This is the first study to quantify the associations between ECG findings and clinical outcome after comprehensive adjustment for clinical factors known to affect outcome. The study's prospective design also allowed for exploration of mechanisms underlying the associations between the ECG variables and clinical outcomes.
The study's results are clinically relevant and should be applicable to the majority of patients with mild to moderate SAH (preoperative WFNS grade 1−3). The pre-operative presence of bradycardia, relative tachycardia, or NSSTTWA identifies patients at risk of poor outcome, independent of other clinical factors. The study's secondary analyses suggest that NSSTTWA, relative tachycardia, and a prolonged QTc interval may identify patients who are more likely to have adverse hemodynamic outcomes during their hospital course, such as post-operative hypotension, and the need for treatment with vasopressors or inotropes for cardiovascular dysfunction, and pulmonary edema. SAH patients with markedly prolonged QTc should probably be followed with increased vigilance for the development of cardiovascular instability. It is notable that a prolonged QTc has been shown to be associated with early cardiac morbidity and mortality after ischemic stroke.15 In the present study, 35% of patients had a prolonged QTc (> 400 msec).
In this study population, the great majority of deaths were on a neurological basis. The associations between the ECG predictors and the need for vasopressors for cardiovascular indications suggest that there is an important link between cardiovascular performance and neurological outcome following aneurysmal SAH. These findings are consistent with other studies which have shown that tachycardia, hypotension, and reduced cardiac index are associated with adverse outcome after SAH, possibly by reducing cerebral perfusion pressure during periods of cerebral vasospasm.16, 17
The ECG findings may not precede cardiac dysfunction, however. It is possible that tachycardia, bradycardia, and SSTWA could be manifestations of neurocardiogenic injury occurring early after SAH. The pathophysiology of neurocardiogenic injury has been well described and appears to be related to excessive cardiac sympathetic stimulation early after brain injury.18-22 In the present study, ECG abnormalities were most commonly observed in the anterior leads, consistent with prior studies which demonstrated that the anterior and anteroseptal walls of the left ventricle are most likely to be affected by neurocardiogenic injury.23, 24
Based on the results of this study, therapeutic options for SAH patients that affect heart rate should be reassessed. On the one hand, beta-blockers may be cardioprotective during the period of high sympathetic outflow early after SAH and may limit relative tachycardia. Older clinical trials suggested a beneficial role for beta-blockers in the care of SAH patients.25, 26 However, the association between bradycardia and mortality in the present study suggests that excessive heart rate lowering could be harmful after SAH. Further clinical research in this area is required.
This study does have limitations. Because all of the patients had SAH, the prevalence of ECG abnormalities could not be compared to a control group, such as patients admitted to the hospital with other non-cardiac conditions. IHAST excluded patients with severe SAH (preoperative WFNS >3), who typically have more neurocardiogenic injury and worse outcomes.8, 27 It is unknown how including these patients would have affected the results. The study included only one ECG per patient and recent studies have suggested that serial ECG acquisition is more sensitive in the detection of ECG abnormalities.28
A large number of ECGs in this study had NSSTTWA in comparison to specific repolarization abnormalities, such as ST depression or T wave inversion. Therefore, there was more statistical power to find associations between NSSTTWA and the outcomes, in comparison to specific abnormalities, which may be more clinically applicable. There was a non-significant association between ST depression and GOS in the multivariate analysis. It is possible but unproven that NSSTTWA, as defined by this study, are a surrogate for any type of repolarization abnormality in predicting adverse outcomes after SAH.
In summary, preoperative bradycardia, relative tachycardia, and NSSTTWA on ECG are independently associated with increased mortality after SAH of mild to moderate severity. These findings add to the growing body of evidence which indicates that there are important relationships between cardiovascular dysfunction and neurological outcomes after SAH. Further mechanistic and trial-based research is required to determine whether these relationships are causal in nature.
Acknowledgements
Bongin Yoo and Qian Shi for biostatistical support. We thank the IHAST Investigators. A full list of investigators and participating centers of IHAST has been published.
Support for this study was provided by the National Institute of Neurological Disease and Stroke (National Institutes of Health) grant RO1 NS38554 to M. M. Todd and departmental funds at the University of California, San Francisco
Footnotes
The authors have no conflicts of interest related to this study
References
- 1.Burch GE, Meyers R, Abildskov JA. A new electrocardiographic pattern observed in cerebrovascular accidents. Circulation. 1954;9:719–723. doi: 10.1161/01.cir.9.5.719. [DOI] [PubMed] [Google Scholar]
- 2.Brouwers PJ, Wijdicks EF, Hasan D, Vermeulen M, Wever EF, Frericks H, van Gijn J. Serial electrocardiographic recording in aneurysmal subarachnoid hemorrhage. Stroke. 1989;20:1162–1167. doi: 10.1161/01.str.20.9.1162. [DOI] [PubMed] [Google Scholar]
- 3.Zaroff J, Rordorf G, Newell J, Ogilvy C, Levinson J. Cardiac outcome in patients with subarachnoid hemorrhage and electrocardiographic abnormalities. Neurosurgery. 1999;44:34–39. doi: 10.1097/00006123-199901000-00013. [DOI] [PubMed] [Google Scholar]
- 4.Sommargren CE, Zaroff JG, Banki N, Drew BJ. Electrocardiographic repolarization abnormalities in subarachnoid hemorrhage. J Electrocardiol. 2002;35(Suppl):257–262. doi: 10.1054/jelc.2002.37187. [DOI] [PubMed] [Google Scholar]
- 5.Schuiling WJ, Algra A, de Weerd AW, Leemans P, Rinkel GJ. Ecg abnormalities in predicting secondary cerebral ischemia after subarachnoid haemorrhage. Acta Neurochir (Wien) 2006;148:853–858. doi: 10.1007/s00701-006-0808-3. discussion 858. [DOI] [PubMed] [Google Scholar]
- 6.Kawasaki T, Azuma A, Sawada T, Sugihara H, Kuribayashi T, Satoh M, Shimizu Y, Nakagawa M. Electrocardiographic score as a predictor of mortality after subarachnoid hemorrhage. Circ J. 2002;66:567–570. doi: 10.1253/circj.66.567. [DOI] [PubMed] [Google Scholar]
- 7.Sakr YL, Lim N, Amaral AC, Ghosn I, Carvalho FB, Renard M, Vincent JL. Relation of ecg changes to neurological outcome in patients with aneurysmal subarachnoid hemorrhage. Int J Cardiol. 2004;96:369–373. doi: 10.1016/j.ijcard.2003.07.027. [DOI] [PubMed] [Google Scholar]
- 8.Hunt W, Hess R. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg. 1967;28:14–20. doi: 10.3171/jns.1968.28.1.0014. [DOI] [PubMed] [Google Scholar]
- 9.Todd MM, Hindman BJ, Clarke WR, Torner JC. Mild intraoperative hypothermia during surgery for intracranial aneurysm. N Engl J Med. 2005;352:135–145. doi: 10.1056/NEJMoa040975. [DOI] [PubMed] [Google Scholar]
- 10.Report of world federation of neurological surgeons committee on a universal subarachnoid hemorrhage grading scale. J Neurosurg. 1988;68:985–986. doi: 10.3171/jns.1988.68.6.0985. [DOI] [PubMed] [Google Scholar]
- 11.Fisher CM, Kistler JP, Davis JM. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery. 1980;6:1–9. doi: 10.1227/00006123-198001000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Wityk RJ, Pessin MS, Kaplan RF, Caplan LR. Serial assessment of acute stroke using the nih stroke scale. Stroke. 1994;25:362–365. doi: 10.1161/01.str.25.2.362. [DOI] [PubMed] [Google Scholar]
- 13.Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet. 1975;1:480–484. doi: 10.1016/s0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- 14.Noordzij PG, Boersma E, Bax JJ, Feringa HH, Schreiner F, Schouten O, Kertai MD, Klein J, van Urk H, Elhendy A, Poldermans D. Prognostic value of routine preoperative electrocardiography in patients undergoing noncardiac surgery. Am J Cardiol. 2006;97:1103–1106. doi: 10.1016/j.amjcard.2005.10.058. [DOI] [PubMed] [Google Scholar]
- 15.Prosser J, MacGregor L, Lees KR, Diener HC, Hacke W, Davis S. Predictors of early cardiac morbidity and mortality after ischemic stroke. Stroke. 2007;38:2295–2302. doi: 10.1161/STROKEAHA.106.471813. [DOI] [PubMed] [Google Scholar]
- 16.Mayer S, Lin J, Homma S, Solomon R, Lennihan L, Sherman D, Fink M, Beckford A, Klebanoff L. Myocardial injury and left ventricular performance after subarachnoid hemorrhage. Stroke. 1999;30:780–786. doi: 10.1161/01.str.30.4.780. [DOI] [PubMed] [Google Scholar]
- 17.Yarlagadda S, Rajendran P, Miss JC, Banki NM, Kopelnik A, Wu AH, Ko N, Gelb AW, Lawton MT, Smith WS, Young WL, Zaroff JG. Cardiovascular predictors of in-patient mortality after subarachnoid hemorrhage. Neurocrit Care. 2006;5:102–107. doi: 10.1385/NCC:5:2:102. [DOI] [PubMed] [Google Scholar]
- 18.Murphy AM, Kogler H, Georgakopoulos D, McDonough JL, Kass DA, Van Eyk JE, Marban E. Transgenic mouse model of stunned myocardium. Science. 2000;287:488–491. doi: 10.1126/science.287.5452.488. [DOI] [PubMed] [Google Scholar]
- 19.White M, Wiechmann R, Roden R, Hagan M, Wollmering M, Port J, Hammond E, Abraham W, Wolfel E, Lindenfeld J. Cardiac beta-adrenergic neuroeffector systems in acute myocardial dysfunction related to brain injury. Evidence for catecholamine-mediated myocardial damage. Circulation. 1995;92:2183–2189. doi: 10.1161/01.cir.92.8.2183. al. e. [DOI] [PubMed] [Google Scholar]
- 20.Mertes P, Carteaux J, Jaboin Y, Pinelli G, el Abassi K, Dopff C, Atkinson J, Villemot J, Burlet C, Boulange M. Estimation of myocardial interstitial norepinephrine release after brain death using cardiac microdialysis. Transplantation. 1994;57:371–377. doi: 10.1097/00007890-199402150-00010. [DOI] [PubMed] [Google Scholar]
- 21.Banki NM, Kopelnik A, Dae MW, Miss J, Tung P, Lawton MT, Drew BJ, Foster E, Smith W, Parmley WW, Zaroff JG. Acute neurocardiogenic injury after subarachnoid hemorrhage. Circulation. 2005;112:3314–3319. doi: 10.1161/CIRCULATIONAHA.105.558239. [DOI] [PubMed] [Google Scholar]
- 22.Zaroff JG, Pawlikowska L, Miss JC, Yarlagadda S, Ha C, Achrol A, Kwok PY, McCulloch CE, Lawton MT, Ko N, Smith W, Young WL. Adrenoceptor polymorphisms and the risk of cardiac injury and dysfunction after subarachnoid hemorrhage. Stroke. 2006;37:1680–1685. doi: 10.1161/01.STR.0000226461.52423.dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zaroff JG, Rordorf GA, Ogilvy CS, Picard MH. Regional patterns of left ventricular systolic dysfunction after subarachnoid hemorrhage: Evidence for neurally mediated cardiac injury. Journal of the American Society of Echocardiography. 2000;13:774–779. doi: 10.1067/mje.2000.105763. [DOI] [PubMed] [Google Scholar]
- 24.Kothavale A, Banki NM, Kopelnik A, Yarlagadda S, Lawton MT, Ko N, Smith WS, Drew B, Foster E, Zaroff JG. Predictors of left ventricular regional wall motion abnormalities after subarachnoid hemorrhage. Neurocrit Care. 2006;4:199–205. doi: 10.1385/NCC:4:3:199. [DOI] [PubMed] [Google Scholar]
- 25.Neil-Dwyer G, Walter P, Cruickshank J, Doshi B, O'Gorman P. Effect of propranolol and phentolamine on myocardial necrosis after subarachnoid haemorrhage. Br Med J. 1978;2:990–992. doi: 10.1136/bmj.2.6143.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neil-Dwyer G, Cruickshank J, Stratton C. Beta-blockers, plasma total creatine kinase and creatine kinase myocardial isoenzyme, and the prognosis of subarachnoid hemorrhage. Surgical Neurology. 1986;25:163–168. doi: 10.1016/0090-3019(86)90287-9. [DOI] [PubMed] [Google Scholar]
- 27.Tung P, Kopelnik A, Banki N, Ong K, Ko N, Lawton MT, Gress D, Drew B, Foster E, Parmley W, Zaroff J. Predictors of neurocardiogenic injury after subarachnoid hemorrhage. Stroke. 2004;35:548–551. doi: 10.1161/01.STR.0000114874.96688.54. [DOI] [PubMed] [Google Scholar]
- 28.Sommargren CE. Electrocardiographic abnormalities in patients with subarachnoid hemorrhage. Am J Crit Care. 2002;11:48–56. [PubMed] [Google Scholar]