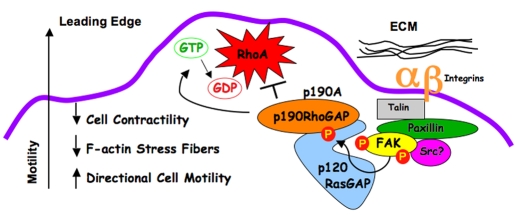
Fig. 10.
Model of the spatial and temporal regulation of p190A by FAK. FAK is activated by integrin clustering and is localized to leading-edge FAs in migrating MEFs via binding to the integrin-associated proteins paxillin and talin. FAK Y397 phosphorylation promotes the SH2-mediated binding of p120RasGAP to FAK and the SH2-SH3-SH2 region of p120RasGAP facilitates a bridge between FAK and p190A. Increased p190A tyrosine phosphorylation is dependent upon FN-stimulated FAK activity that can either phosphorylate p190A directly (Holinstat et al., 2006) or promote p190A tyrosine phosphorylation via Src activation (Wu et al., 2008). In cells with activated Src, cell polarity and recruitment of p190A to leading-edge paxillin-containing FAs is dependent upon FAK expression. Increased GAP activity of tyrosine-phosphorylated p190A that is localized to FAs acts to inhibit RhoA GTPase activity, and this is associated with decreased actin contractility at leading-edge lamella and enables directional or polarized cell movement.