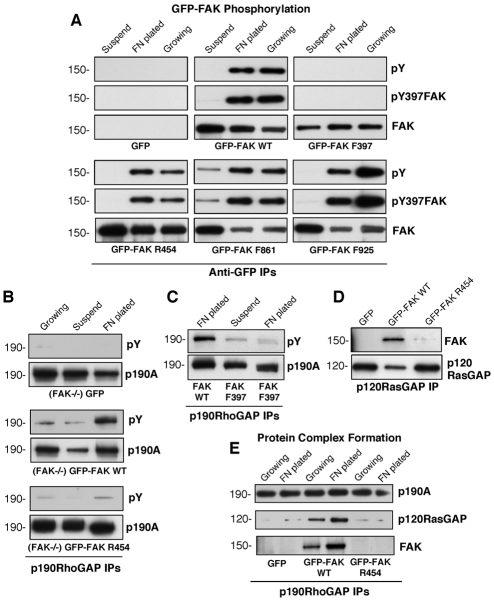
Fig. 9.
FAK activity facilitates p190A tyrosine phosphorylation and complex formation with p120RasGAP and p190A. (A) Regulation of GFP-FAK phosphorylation in reconstituted FAK–/– MEFs. Lysates were made from starved cells held in suspension (1 hour), cells that were replated onto FN-coated dishes (45 minutes) and from growing cells. Anti-GFP IPs were sequentially analyzed by anti-pY, FAK pY397 phospho-specific and total FAK blotting. (B) p190A tyrosine phosphorylation is dependent upon FAK activity. p190A IPs were made from growing, suspended or FN-plated (30 minutes) FAK–/– MEFs expressing GFP or reconstituted with GFP–FAK-WT or GFP–FAK-R454 and analyzed by anti-pY followed by anti-p190A immunoblotting. (C) Mutation of FAK Y397 blocks p190A tyrosine phosphorylation. p190A IPs from suspended or FN-plated FAK–/– MEFs expressing GFP–FAK-WT or GFP–FAK-F397 were analyzed by anti-pY followed by anti-p190A immunoblotting. (D) FAK activity is required for p120RasGAP association. p120RasGAP IPs from FN-plated FAK–/– MEFs expressing GFP or reconstituted with GFP–FAK-WT or GFP–FAK-R454 were analyzed for FAK association by anti-FAK followed by anti-p120RasGAP immunoblotting. (E) p190A-p120RasGAP-FAK association is dependent upon FN-stimulated FAK activity. p190A IPs from growing or FN-plated FAK–/– MEFs expressing GFP or reconstituted with GFP–FAK-WT or GFP–FAK-R454 were analyzed for p120RasGAP and FAK association by sequential immunoblotting.