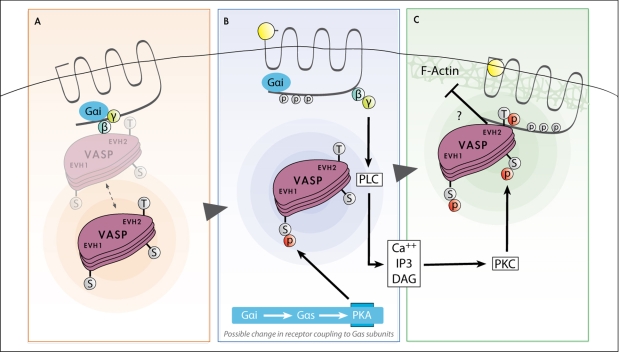
Fig. 12.
Model illustrating the roles of VASP phosphorylation in mediating the VASP-CXCR2 interaction. (A) CXCR2 and VASP in unstimulated cells. VASP is predominantly located in the cytoplasm but might have some loose basal association with CXCR2. (B) Upon ligand binding, uncoupling of the G proteins occurs, activating PKA- and PKC-signaling pathways. VASP localizes to the plasma membrane. It is likely that there is a change in Gα coupling to CXCR2 to activate PKA, which phosphorylates VASP on Ser157 in the EVH1 domain. (C) Uncoupling of the Gβγ subunit activates PKC, which phosphorylates Ser239 and possibly Thr278 in the EVH2 domain of VASP. These phosphorylations allow strong interaction between VASP and CXCR2. CXCR2 binding to VASP is likely mutually exclusive with the F-Actin–VASP interaction but it is possible that when phosphorylated VASP is bound to CXCR2, sustained interaction with F-actin occurs.