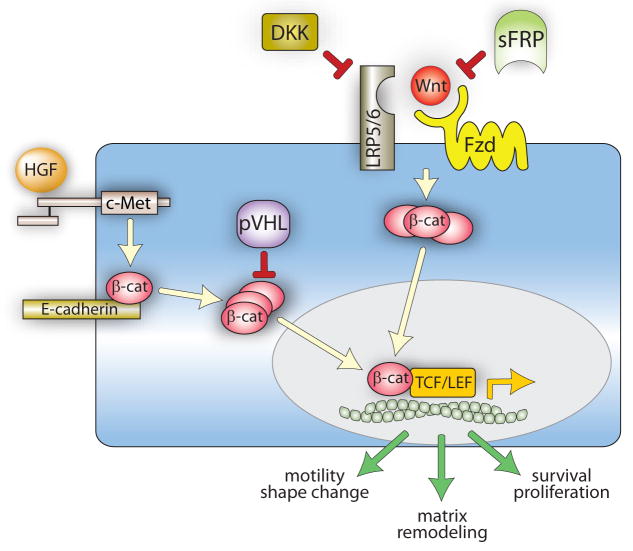
Figure 1. Hypothetical model of HGF and Wnt signaling in kidney development and adulthood.
During development, HGF-Met binding promotes receptor kinase activation and tyrosyl phosphorylation of junctional β-catenin, resulting in its dissociation from E-cadherin and cytoplasmic accumulation. Wnt binding to Fzd and LRP5/6 induces Fzd activation, which stabilizes cytoplasmic β-catenin by protecting it from constitutive degradation. Cytoplasmic β-catenin from either pathway translocates to the nucleus, where it associates with members of TCF/LEF transcription factor family and promotes the expression of genes controlling cell motility, proliferation and matrix remodeling. In adult, fully differentiated renal epithelial cells, pVHL targets cytosolic β-catenin for degradation and balanced expression of Wnts and the negative regulators DKKs and sFRPs keeps Fzd activation, and thus cytosolic β-catenin, to a minimum.