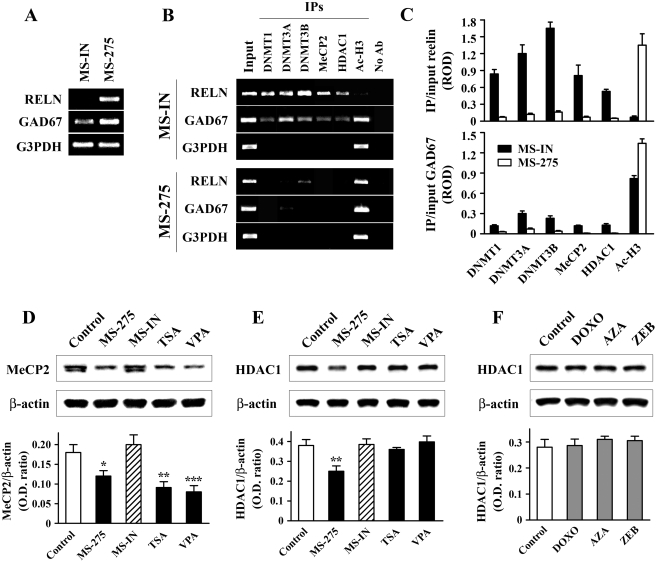
Fig. 6.
Reelin and GAD67 gene activation is accompanied by dissociation of repressor complexes from the corresponding promoters. NT-2 cells were treated with either 5 μM MS-IN or 5 μM MS-275 for 48h. A, RT-PCR analysis confirmed that reelin and GAD67 mRNAs were induced by MS-275. B, the corresponding chromatin preparations were immunoprecipitated with DNMT1, DNMT3A, DNMT3B, MeCP2, HDAC1, and Ac-H3 antibodies (IPs). Nonimmunoprecipitated samples were used as negative controls (No Ab). DNA isolated from inputs, IPs, and control samples were PCR-amplified using primers specific for the reelin, GAD67, and G3PDH promoter regions. Relative optical densities (RODs) of the bands derived from ethidium bromide-stained gels were quantified. C, graphs show the results (mean ± S.E.M of three independent experiments) of semiquantitative analysis of the occupancy of DNMT1, DNMT3A, DNMT3B, MeCP2, HDAC1, and Ac-H3 on the reelin (top) and GAD67 (bottom) promoters in MS-IN- and MS-275-treated cells, normalized to input DNA (each comparison MS-IN versus MS-275 showed statistical significance of at least p < 0.05, t test). In addition, Western blot analyses of MeCP2 protein after HDAC inhibitor treatment (D), and HDAC1 protein after treatments with HDAC inhibitors (E) and DNMT inhibitors (F) are shown. Drug treatments were as follows: 5 μM MS-275 (48 h), 5 μM MS-IN (48 h), 0.3 μM TSA (24 h), 5 mM VPA (24 h), 250 nM DOXO (48 h), 5 μM AZA (48 h), and 500 μM ZEB (48 h followed by 48-h incubation with untreated medium). In all three cases (D-F), top panels show the representative Western blots, whereas bottom panels show the graphs with the corresponding ratios of the MeCP2 or HDAC1 band to the area of the ß-actin band. All results (D-F, lower panels) are expressed as mean ± S.E.M of three independent experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.001 versus control group (one-way ANOVA followed by Bonferroni test).