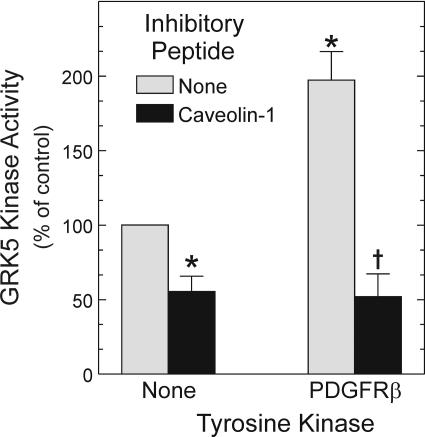
Fig. 10.
Caveolin-1 abrogates the increase in GRK5 activity resulting from PDGFRβ-mediated tyrosyl phosphorylation. GRK5 substrate peptide phosphorylation assays were performed as in Fig. 8, but in two sequential stages: 1) nonradioactive phosphorylation, in which GRK5 was incubated with immune complexes from PDGFRβ-expressing or -deficient (control) HEK cells; and 2) [γ-32P]ATP phosphorylation, for which GRK5 was separated from immune complexes before combining with GRK5 substrate peptide, in the presence or absence of caveolin-1 (or -2) scaffolding domain peptides, as described under Materials and Methods. GRK5 substrate peptide cpm were normalized to those obtained with GRK5 that had not been prephosphorylated by the PDGFRβ and was not incubated with caveolin peptides (control), to obtain the percentage of control. Shown are the means ± S.E. from four independent experiments. Compared with control: *, p < 0.05. Compared with the cognate reaction conducted without caveolin-1 peptide: †, p < 0.05.