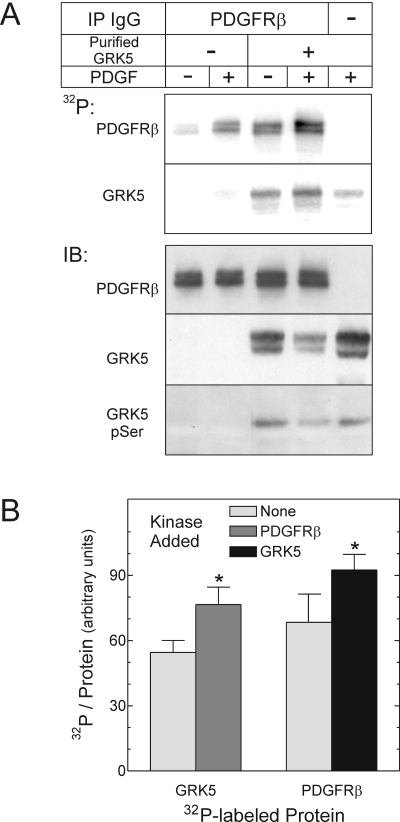
Fig. 4.
The PDGFRβ tyrosine phosphorylates GRK5 stoichiometrically. PDGFRβs were immunoprecipitated from HEK cells exposed to medium containing vehicle (-) or 2 nM PDGF-BB (+) for 10 min at 37°C. After IP, purified GRK5 was added to PDGFRβ immune complexes, and kinase assays proceeded with [γ-32P]ATP. Samples were resolved by SDS-PAGE and transferred to nitrocellulose, as described under Materials and Methods.A, 32P autoradiograms from a single experiment, representative of four performed, are presented at the top. Bottom, IBs performed on autoradiographed nitrocellulose were probed serially for phosphoserine (pSer), GRK5, and the PDGFRβ; images from a single experiment represent four performed. B, 32P band intensities in lanes from PDGF-stimulated cells were quantitated by PhosphorImager and divided by the cognate IB band densities for the PDGFRβ and GRK5. Reactions were performed with purified GRK5 or the PDGFRβ, in the absence (None) or presence of each other, as in A. Shown are the means ± S.E. of four independent experiments. Compared with values obtained in reactions with a single kinase: *, p < 0.05.