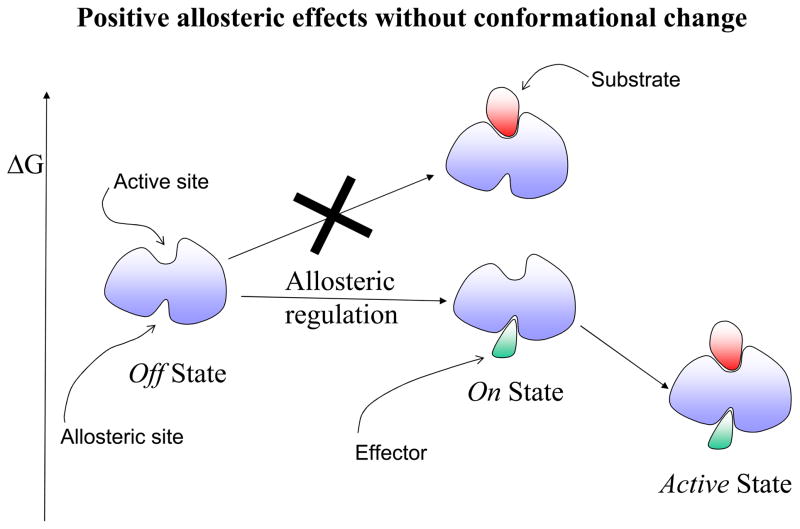
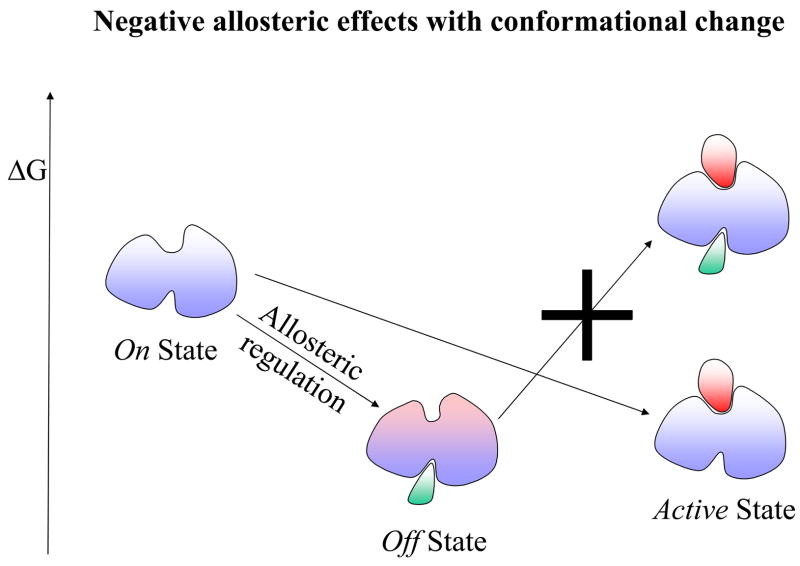
Figure 1.
A schematic drawing illustrating the allosteric effect. Here the allosteric effect is referred to as a change at one site (allosteric site) affecting the activity of another site (active site). In this drawing, the activity at both sites is depicted as a ligand binding event: an effector binding to the allosteric site and a substrate binding to the active site. The location of the two binding sites could be adjacent or distal (as shown in the drawing) to each other. The allosteric effect is said to show positive cooperativity if the effector binding increases the affinity for the substrate (favorable binding free energy). Conversely, if the effector binding lowers the substrate affinity, it exhibits negative cooperativity. The change at the allosteric site (an effector binding in the drawing) might, or might not, alter the conformation at the active site. Therefore, there are four combinations of allosteric effects in terms of positive/negative cooperativity versus with/without conformational changes. Here we illustrate only two of these cases in terms of the relative free energy change: positive allosteric regulation without conformational change in Figure 1A; and negative allosteric regulation with conformational change in Figure 1B. In positive allosteric regulation (Figure 1A), the event of the effector binding at the allosteric site (indicated as Allosteric regulation) switches it from an Off regulation state to an On regulation state. Note that the discrete On or Off regulation state in the Figure is highlighted in order to reflect the outcome of the cellular regulation functionality. For a general definition of the allosteric effect, the degree of change is always continuous either increasing or decreasing the affinity at the substrate site. The allosteric binding clearly shows that the previous unfavorable substrate binding for the Off state (indicated by a big cross) becomes favorable for the On state. In negative allosteric regulation (Figure 1B), an effector binding at the allosteric site (also indicated as an Allosteric regulation) switches it from an On regulation state to an Off regulation state. Here, the allosteric binding indicates that the previous favorable substrate binding for the On state becomes unfavorable (indicated by a big cross) for the Off state. The conformational change due to the effector binding is highlighted in pink color around the active site.