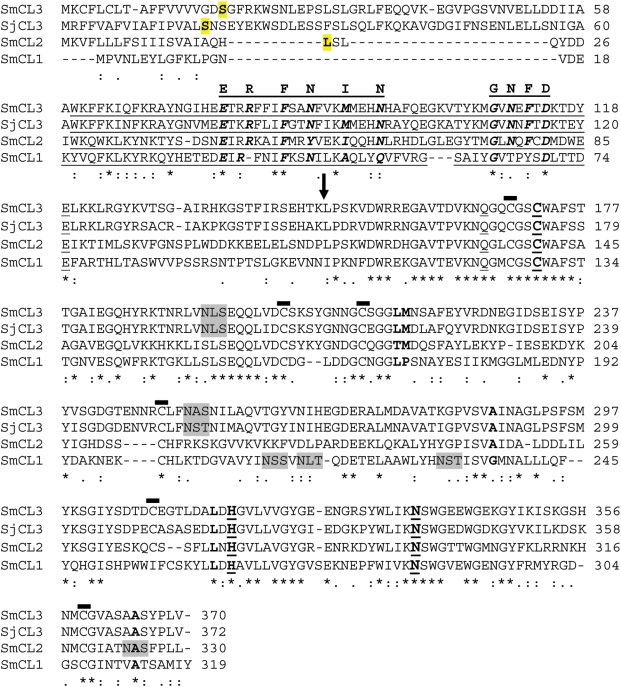
Figure 1. Multiple alignment of SmCL3 (ABV71063) with orthologous SjCL3 from S. japonicum (AAW27185) and characterized S.mansoni cathepsin L-like genes (SmCL2, CAA83538; SmCL1, Q26534).
The catalytic triad residues (C, H and N) are marked in bold and underlined. Glutamine involved in the formation of the oxyanion hole and preceding the catalytic cysteine is underlined. Potential N-linked glycosylation sites are shaded in grey. The predicted starts of the pro-peptide and catalytic domains are highlighted in bold and shaded in yellow, and by an arrow, respectively. Type I-29 protease inhibitor is underlined. ERFNIN and GNFD motifs present in the pro-peptide are overlined with amino acid residues highlighted in bold italic. Six cysteines forming three putative disulfide bonds that are present the catalytic domain are marked by bold overlines. Residues forming the critical S2 subsite specificity pocket are in bold.