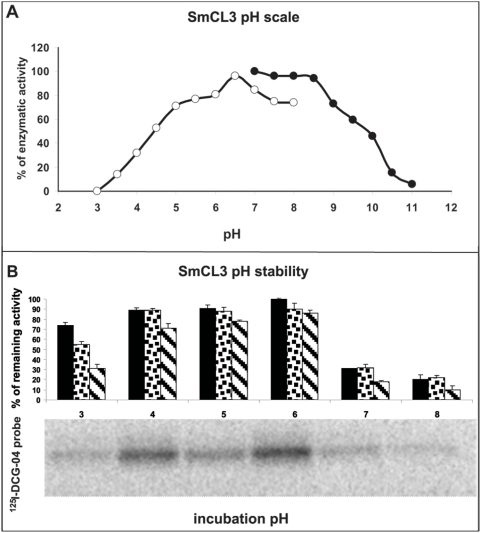
Figure 4. pH optimum and stability of recombinant SmCL3.
(A) Using the fluorogenic peptidyl substrate, Z-Phe-Arg-AMC, SmCL3 demonstrates a broad pH optimum. Enzyme was assayed in 50 mM citrate, 100 mM sodium phosphate buffer (○) or 100 mM glycine buffer (•), both containing 2 mM DTT. (B) Peptidolytic activity was measured with Z-Phe-Arg-AMC in 50 mM citrate, 100 mM sodium phosphate, 2 mM DTT, pH 6.0, after preincubation under different pH conditions in 50 mM citrate, 100 mM phosphate for 30 min (black bars), 1 h (dotted bars) and 3 h (striped bars). Samples that had been incubated for 3 h were also labeled with the active site affinity probe 125I-DCG-04 (amount of active enzyme visualized corresponds to the amount measured with Z-Phe-Arg-AMC).