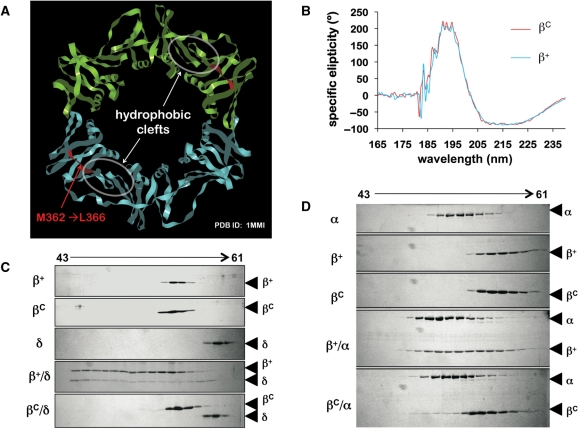
Figure 1.
Deletion of residues M362-L366 in the β clamp impairs the cleft without affecting the overall tertiary structure of the clamp protein. (A) Proximity of residues M362-L366 to the hydrophobic cleft in the β clamp is indicated. This structural figure was generated using imol and the coordinates for the wild type β clamp (1MMI) from the PDB. (B) Averaged results of circular dichroism spectroscopy are shown from four separate scans. Representative results from Superose 12 gel filtration chromatography examining (C) interactions of β+ and βC (2.5 μM as dimer) with the δ subunit of DnaX complex (5 μM), or (D) the α catalytic subunit of Pol III (Pol IIIα; 1.5 μM). Untagged forms of recombinant β+ and βC were used in ITC and gel filtration experiments.