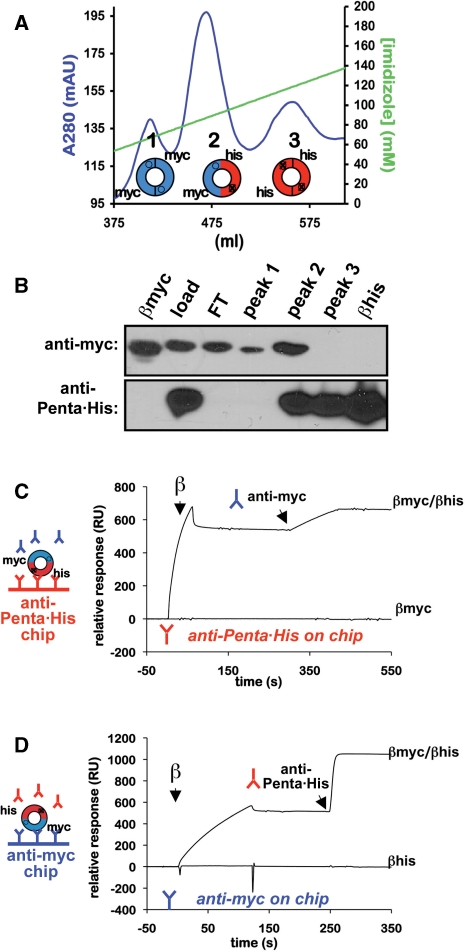
Figure 2.
Purification of heterodimeric β clamp proteins. (A) FPLC chromatography trace of a representative HiTrap cobalt chelating HP column run for myc-β+/his6-β+. Respective positions of peaks 1–3, as well as the type of clamp present in that peak (homodimeric or heterodimeric) are indicated. Myc-tagged clamps are blue, and his6-tagged clamps are red. (B) Western blot analysis of peaks 1–3. Aliquots of each peak were separated by 12% SDS–PAGE, and probed with either anti-myc (top panel) or anti-Penta•His antibody (bottom panel). The load (load), cobalt column flow through (FT), and purified myc- (βmyc) and his6-tagged β+ clamp proteins (βhis) were included as controls. Representative SPR traces in which the his6-tagged subunit was captured on the chip surface using anti-Penta•His antibody (anti-Penta•His on chip), and anti-myc antibody was injected over the chip surface (C), or the myc-tagged clamp subunit was captured on the chip surface using anti-myc antibody and anti-Penta•His was injected (anti-myc on chip) (D) are shown. SPR analysis was performed as described in ‘Materials and Methods’ section. Although representative results for the β+/β+ heterodimer are shown, identical results were observed during purification of the myc-β+/his6-βC heterodimer (β+/βC).