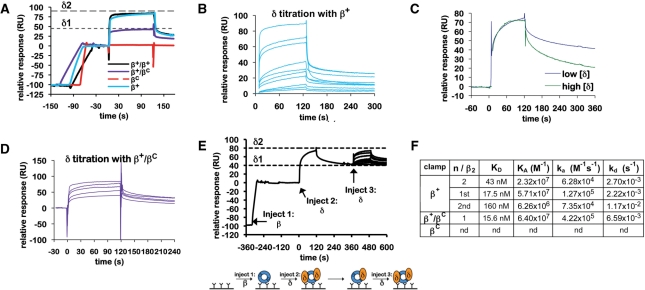
Figure 3.
Interactions of the β clamp with the δ subunit of DnaX. (A) Representative SPR results analyzing interaction of δ (4 μM) with various β clamp proteins (∼100 RU). Clamps examined include the his6-tagged β+ homodimer (β+), the his6-tagged βC homodimer (βC), the his6-tagged β+/myc-tagged β+ heterodimer (β+/β+) and the his6-tagged β+/myc-tagged βC heterodimer (β+/βC). Theoretical Rmax values for binding of a single δ subunit (δ1), or for two δ subunits (δ2) are indicated by dashed lines. (B) Titration of δ with his6-tagged β+. Increasing concentrations of purified δ (0.1, 1, 10, 25, 50, 100, 250, 500, 750 and 1000 nM) were injected over ∼100 RU of his6-tagged β+ captured on the anti-Penta•His chip surface. (C) Comparison of sensorgrams from low (25 nM) and high (1 µM) concentrations of δ injected over ∼100 RU of his6-tagged β+ captured on the anti-Penta•His chip surface from panel (B) highlighting differences in association (ka) and dissociation rates (kd). The amplitudes (Rmax) of these SPR traces were normalized to each other to facilitate a direct comparison of the association and dissociation phases of the curves. (D) Titration of δ with β+/βC. Increasing concentrations of purified δ (10, 25, 50, 100 and 500 nM) were injected over ∼100 RU of β+/βC captured on the anti-Penta•His chip surface. (E) The double-injection experiment was performed as outlined in the cartoon (bottom). ∼100 RU of his6-tagged β+ was captured on the anti-Penta•His chip surface (injection 1). Saturating levels (10 µM) of δ were injected (injection 2), forming the β–δ2 complex (Rmax approaching 100 RU). Flow was switched to buffer, allowing for the more loosely bound δ (δ2) to dissociate, resulting in a β–δ1 complex. Increasing concentrations of δ (0, 1, 10, 25, 50, 100, 250, 500, 750 and 1000 nM) were then injected over the β–δ1 complex (injection 3) to determine the binding affinity for the second δ (δ2). (F) Summary table of kinetic constants for various clamp–δ interactions derived from SPR results summarized in (A–E). Results shown for β+–δ were determined using the his6-tagged β+ homodimer, and were indistinguishable from those observed with β+/β+. An interaction of βC with δ was not detected (nd).