Figure 5.
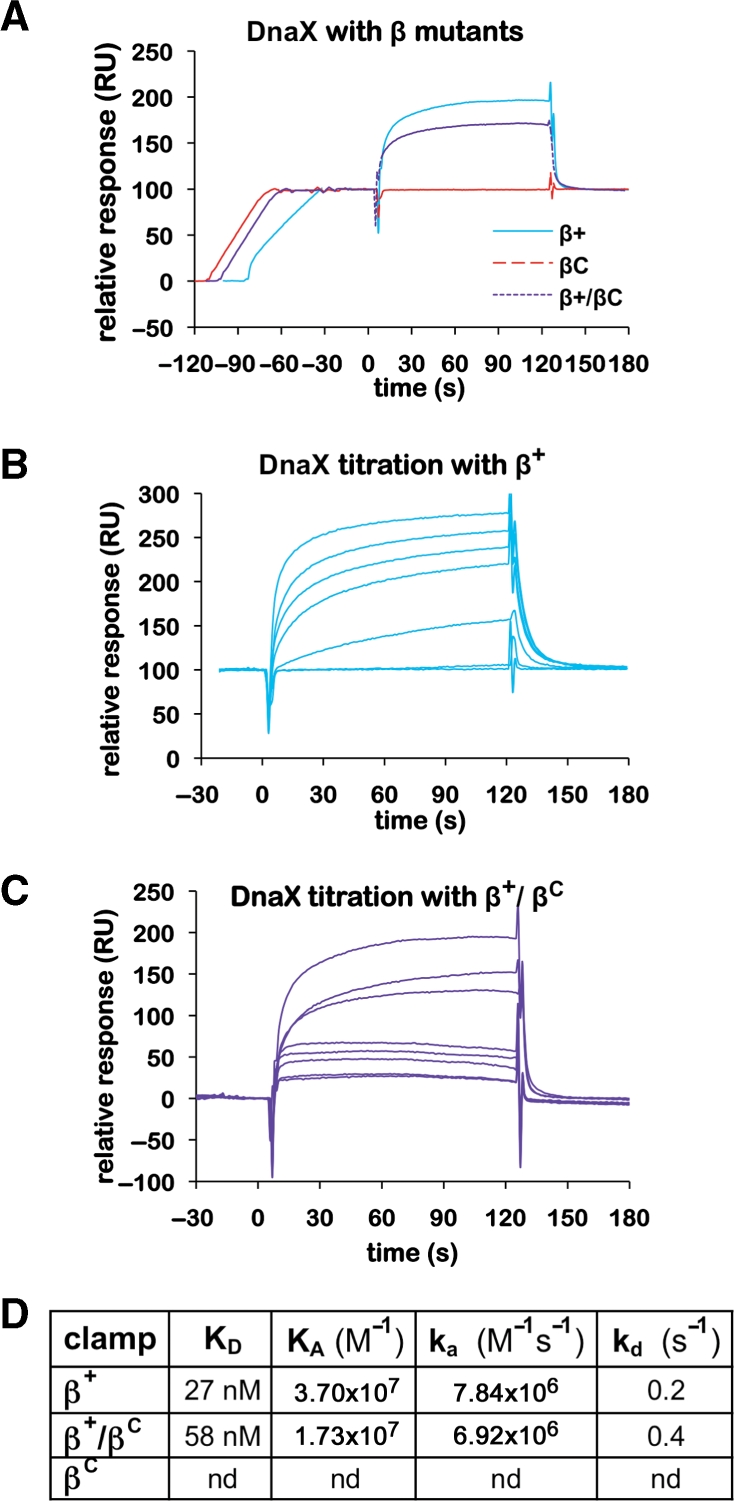
Interactions of wild-type and mutant clamps with the DnaX complex. (A) Representative SPR results analyzing interaction of DnaX (100 nM) with various β clamp proteins (∼100 RU). Clamps examined include the his6-tagged β+ homodimer (β+), the his6-tagged βC homodimer (βC) and the his6-tagged β+/myc-tagged βC heterodimer (β+/βC). (B) Titration of DnaX with β+. Increasing concentrations of the DnaX complex (0, 1, 10, 50, 100, 200 and 500 nM) were injected over ∼100 RU of his6-tagged β+ captured on the anti-Penta•His chip surface. (C) Titration of DnaX with β+/βC. Increasing concentrations of the DnaX complex [1 (in duplicate), 10, 50, 100, 500 (in duplicate) and 1000 nM] were injected over ∼100 RU of β+/βC captured on the anti-Penta•His chip surface. (D) Summary table of kinetic constants for various clamp–DnaX interactions derived from SPR results summarized in (B) and (C). The KD for the DnaX–β+ interaction was reported previously (11), and is included here for direct comparison to βC and β+/βC. An interaction of βC with DnaX was not detected (nd) by SPR.
