Abstract
We report that the protein tyrosine phosphatase PTP-PEST is expressed in resting human and mouse CD4+ and CD8+ T cells, but not in Jurkat T leukemia cells, and that PTP-PEST protein, but not mRNA, was dramatically down-regulated in CD4+ and CD8+ primary human T cells upon T cell activation. This was also true in mouse CD4+ T cells, but less striking in mouse CD8+ T cells. PTP-PEST reintroduced into Jurkat at levels similar to those in primary human T cells, was a potent inhibitor of TCR-induced transactivation of reporter genes driven by NFAT/AP-1 and NF-κB elements and by the entire IL-2 gene promoter. Introduction of PTP-PEST into previously activated primary human T cells also reduced subsequent IL-2 production by these cells in response to TCR and CD28 stimulation. The inhibitory effect of PTP-PEST was associated with dephosphorylation the Lck kinase at its activation loop site (Y394), reduced early TCR-induced tyrosine phosphorylation, reduced ZAP-70 phosphorylation and inhibition of MAP kinase activation. We propose that PTP-PEST tempers T cell activation by dephosphorylating TCR-proximal signaling molecules, such as Lck, and that down-regulation of PTP-PEST may be a reason for the increased response to TCR triggering of previously activated T cells.
Keywords: Protein Tyrosine Phosphatase (PTP), PTP-PEST, PTPN12, Lck, T cell activation
Introduction
Antigen recognition by T lymphocytes results in a rapid increase in tyrosine phosphorylation of numerous cellular proteins (Klausner and Samelson, 1991), initiating several signaling cascades and the formation of a highly organized macromolecular structure at the contact area between the T cell and the APC, referred to as the ‘immunological synapse’ (Monks et al., 1998; Stinchcombe et al., 2001). The molecular machinery that drives immunological synapse formation is incompletely understood, but clearly involves TCR-coupled tyrosine kinases (PTKs) of the Src and Syk families, the tyrosine phosphorylation of a subset of signaling proteins, cholesterol- and glycolipid-enriched lipid rafts, and the actin-based cytoskeleton (Mustelin et al., 2002; Schlaepfer and Hunter, 1998). Since the tyrosine phosphorylation of signaling proteins is dynamic and transient, it appears that protein tyrosine phosphatases (PTPs) also play a key role in TCR signaling (Mustelin et al., 2005) and the formation of an immunological synapse. Indeed, our recent work with the Yersinia phosphatase YopH demonstrated that PTPs can completely prevent both of these responses to antigen (Tautz et al., 2005). However, the identities of the endogenous PTPs that regulate the T cell’s response to antigen are still only partly known.
It should be emphasized that PTPs have a high degree of substrate specificity in vivo, although there are examples of overlapping functions between closely related PTPs. The many PTPs that have been reported to participate in TCR-induced signaling pathways do so in unique and specific ways (Mustelin et al., 2005). For example, HePTP dephosphorylates Y185 in the activation loop of Erk and the equivalent site on p38 kinase, but does not dephosphorylate any other signaling molecules (Saxena et al., 1999a, b; Saxena and Mustelin, 2000; Nika et al., 2006). PTPH1 has been proposed to dephosphorylate the TCR-ζ (Sozio et al., 2004) and VCP (Zhang et al., 1999) (although both remain unverified in T cells). In our hands, SHP1 efficiently dephosphorylates Y493 of ZAP-70, but has minimal effects on Src family kinases (Brockdorff et al., 1999), while LMPTP-B has a positive effect on TCR signaling by dephosphorylating ZAP-70 at the inhibitory site Y292 (Bottini et al., 2002). In addition, even high levels of overexpression of several PTPs (e.g. TCPTP or PTP-MEG2) have no effect at all on TCR signaling (Gjörloff-Wingren et al., 2000).
A candidate PTP for a regulatory role in immunological synapse formation and T cell activation is PTP-PEST, encoded by the PTPN12 gene. In non-lymphoid cells, PTP-PEST participates in the dynamic regulation of focal adhesions, integrin signaling, and Rac-dependent motility (Angers-Loustau et al., 1999; Sastry et al., 2002). In B cells, PTP-PEST, was reported to reduce the phosphorylation of Shc, Pyk2, Fak, and Cas, and to inhibit activation of the Ras pathway (Davidson and Veillette, 2001). In T cells, PTP-PEST was found to dephosphorylate the Wiscott Aldrich syndrome protein (WASP) (Badour at el., 2004). However, the physiological role of PTP-PEST in T cells remains poorly understood, particularly since homozygous deletion of the PTP-PEST gene in mice was embryonically lethal (Cote et al., 1998) and lineage-specific conditional gene deletions have not yet been reported.
PTP-PEST belongs to a group of three PTPs, which also contains LYP (PTPN22), and PTP-HCSF (PTPN18). They all contain an N-terminal PTP domain and an extended C-terminus with several proline-rich motifs (Mustelin et al., 2005). Through one of these motifs, PTP-PEST associates with the SH3 domain of the Csk kinase (Davidson et al., 1997), through another one with the cytoskeletal scaffold protein paxillin (Cote et al., 1999; Shen et al., 1998, 2000), and through the C-terminal region with PSTPIP1 (Spencer et al., 1997), and PSTPTP2 (Wu et al., 1998). All three proteins appear to be dephosphorylated by PTP-PEST and PSTPIP1 reportedly also directs PTP-PEST towards the c-Abl tyrosine kinase (Cong et al., 2000) and WASP (Badour et al., 2004; Cote et al., 2002). Other reported substrates for PTP-PEST in non-lymphoid cells include Cas (Cote et al., 1998; Garton et al., 1996), Pyk2 and Fak (Lyons et al., 2001). Since many of these same proteins are involved also in integrin signaling and assembly of the immunological synapse in lymphocytes (Manie et al., 1997), it seems likely that PTP-PEST may have a major impact on antigen recognition and T cell activation.
PTP-PEST was also found in a screening assay for proteins that could rescue yeast from the lethality of c-Src (Superti-Furga et al., 1996), and was the only protein that reduced the phosphorylation of Src at its positive regulatory site, Y416. This function as a Src PTK suppressor is compatible with the physical association of PTP-PEST with Csk and may suggest that some of the effect of PTP-PEST on focal adhesion proteins is indirect and is mediated by inactivation of Src family PTKs. Here, we have begun to address the role of PTP-PEST in T cells by verifying that it is indeed present in human T cells and by following its expression during T cell activation. We find that PTP-PEST is rapidly downregulated by TCR stimulation of naïve human or mouse T cells. Previously activated T cells, which lack PTP-PEST, are known to respond much more vigorously to TCR restimulation. Expressing PTP-PEST in these cells reverted them to the reduced responsiveness of naïve T cells. The highly TCR responsive T leukemia cell line Jurkat is also negative for PTP-PEST protein and mRNA and transfection of PTP-PEST into these cells resulted in reduced activation of Lck, much less robust tyrosine phosphorylation of cellular proteins, and diminished downstream responses. Together, all these data suggest that PTP-PEST acts as a gatekeeper of TCR signaling and that its downregulation upon activation of naïve T cells may be a key feature of the molecular mechanisms that underlie the rapid and increased responsiveness of previously activated (‘memory’) T cells.
2. Materials and Methods
2.1 Antibodies and reagents
Rabbit anti-PTP-PEST Ab (Angers-Loustau et al., 1999) was a kind gift from Dr. J.-F. Cote (Clinical Research Institute of Montreal, Canada). Rabbit and mouse anti-PTP-PEST Abs were purchased from Orbigen (San Diego, CA) and Sigma-Aldrich Corp. (clone AG25: St. Louis, MO), respectively. Anti-influenza hemagglutinin (HA) mAb (12CA5) was purchased from Roche Applied Science (Indianapolis, IN). The 4G10 anti-pTyr mAb was from Chemicon/Upstate (Temecula, CA). Anti-human CD3ε mAb (OKT3) was from eBioscience (San Diego, CA), anti-CD28 was from BD Biosciences (San Jose, CA). Goat anti-mouse IgG Ab was from Jackson ImmunoResearch (West Grove, PA). Polyclonal anti-phosho-Y394-Lck (anti-phospho-Y416-Src), anti-phosho-Y319-, and phospho-Y493-ZAP-70 were from Cell Signaling Technology Inc. (Beverly, MA), anti-phospho-Erk and anti-phospho-p38 were from Promega (Madison, WI). The polyclonal anti-Erk2, anti-Csk, and anti-LAT were from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-ZAP-70 was from Zymed Laboratories Inc. (San Francisco, CA). Rabbit polyclonal anti-actin was from Sigma-Aldrich Corp. Both anti-human and mouse CD3/CD28 Abs-coated beads were from Dynal Biotech (Oslo, Norway). Anti-human CD45RA (HI100), PE-anti-human CD45RA (JS-83), anti-human CD45RO (UCHL1), FITC-anti-human CD4 (OKT4), PE-anti-human CD8 (OKT8), anti-human CD4 (RPA-T4), anti-human CD8 (RPA-T8), and anti-human CD41 (HIP8) were purchased from eBioscience (San Diego, CA). FITC-anti-CD45RO was from Caltag laboratories (Burlingame, CA). Dynabeads Pan Mouse IgG was from Dynal Biotech (Oslo, Norway). p-nitrophenyl phosphate (pNPP) was purchased from Sigma (St. Louis, MO).
2.2 PCR for PTP-PEST expression
PCR for PTP-PEST expression was conducted using multiple tissues cDNA libraries (BD Biosciences Clontech, Mountain View, CA). The primer pairs used are as follows: for PTP-PEST, 5′-GATGGTGCTGTGACCAGGAAC-3′ for sense and 5′-TCATGTCCATTCTGAAGGTGG -3′ for anti-sense; for G3PDH, 5′-CCATCACCATCTTCCAGGAGC-3′ for sense and 5′-CACCACCTTCTTGATGTCATC-3′ for anti-sense. The PCR reaction was at 94°C for 30 sec, 50°C for 30 sec, and 72°C for 45 sec, 30 cycles, followed by 10 min at 72°C and then resolved by 1% agarose gel.
2.3 Expression plasmids
Wild-type and enzymatically-inactive mutant (C231S) of mouse PTP-PEST were cloned into the expression vectors pEF-BOS and pcDNA3.1 (Angers-Loustau et al., 1999). Human LYP, PTPH1, SHP1, LMPTP-B and HePTP cDNA in mammalian expression vector pEF5HA were described previously (Bottini et al., 2002; Brockdorff et al., 1999; Gjörloff-Wingren et al., 2000; Saxena et al., 1998; Vang et al., 2005). The HD-PTP cDNA was a gift from Dr. M. Ouchida (Okayama University, Japan; Toyooka et al., 2000)
2.4 Cells and transfection
Jurkat T cells were kept at logarithmic growth in RPMI 1640 medium supplemented with 10% fetal calf serum, 2 mM L-glutamine, and 100 units/ml each of penicillin G and streptomycin. Electroporation conditions typically contained 20 × 106 Jurkat T cells and a total of 10–20 μg of plasmid DNA in 0.4 ml Opti-MEM I (Gibco-Invitrogen) and was conducted by BTX T820 electroporator (Harvard Apparatus, Inc., Holliston, MA) at 230 V, 65 msec, 1 pulse, and in each transfection the DNA amount was kept constant by the addition of empty vector. Cells were used for experiments within 24–36 h after transfection.
Human T cells were isolated from peripheral blood of healthy volunteers, purchased from the San Diego Red Cross Blood Service, by Ficoll gradient centrifugation with RosetteSep Human T cell Enrichment Cocktail (Stemcell Technologies) or by eliminating monocytes and B cells with anti-CD14 and anti-CD19 beads (Dynal Biotech) for 30 min at 4 °C. The purity of T cells was >93%. Transient transfection of normal human T cells was done by using human T cell Nucleofector kit according to manufacture’s instruction (Amaxa GmbH, Cologne, Germany). For further purification, T cells prepared as above were treated to remove residual red blood cells with lysing buffer (Sigma), washed with PBS, and incubated with anti-CD41 (to remove residual platelets) plus anti-CD4 or CD8 Abs on ice, then separated negatively with magnetic beads (Dynal Biotech). For naïve and memory T cell purification, T cells were incubated with anti-CD45RA or CD45RO Abs, then separated either positively or negatively. The purity was confirmed by FACS using fluorescence-conjugated Abs that were different clones from those used for cell separation to recognize different epitopes of each molecule. C57BL/6 mice were maintained in the Burnham Institute colony. Splenic CD4 and CD8 T cells were prepared using MACS mouse T cell isolation kits according to manufacturer’s instruction (Miltenyi Biotec, Auburn, CA).
2.5 T cell stimulation and immunoblotting
Jurkat T cells were stimulated by adding 10 μl/ml of OKT3 to the cells for the indicated times at 37 °C. For stimulation of primary T cells, the cells were first incubated with 10 μg/ml of OKT3 in ice for 10 min, spun down, and then incubated with 15 μg/ml of a cross-linking goat anti-mouse Ig at 37 °C. The stimulation was stopped by adding ice-cold phosphate-buffered saline and the cells were pelleted and lysed in TNE cell lysis buffer (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 5 mM EDTA containing 1% Nonidet P-40, 1 or 5 mM Na3VO4, and 1 mM phenylmethylsulfonyl fluoride) on ice for 10 min and clarified by centrifugation at 12,000 rpm for 3 min. The lysates were mixed with 4× SDS sample buffer, boiled for 2 min, and resolved by SDS-PAGE. All immunoblots were developed by the enhanced chemiluminescence technique (ECL kit, Amersham, Piscataway, NJ). For long-term stimulation, T cells were incubated with beads coated with anti-CD3 and anti-CD28 (Dynal Biotech) at a 1:1 ratio (2 × 106 : 2 × 106) in 24-well plates for 2–3 days. The activated T cells were then incubated/rested in the presence of 100 U/ml of human rIL-2 (Peprotech Inc., Rocky Hill, NJ) for another 2 days and then re-stimulated with the same beads for 8–24 h. The cell lysates prepared as above were subjected to western blot analysis.
2.6 Luciferase assay and IL-2 secretion
Jurkat cells were co-transfected with luciferase reporter constructs (IL-2-luc, NF-κB-luc, or NFAT/AP-1-luc) and PTP-PEST expression vector or empty vector by electroporation as above. The next day, cells were stimulated with anti-CD3 and anti-CD28 in conjunction with anti-mouse Ig for 24 h. The cell lysates were used to measure luciferase activity using the Dual Reporter System kit from Promega. For IL-2 secretion assays, 2 × 106 normal human T lymphocytes that had been transfected with PTP-PEST expression vector by nucleofection were stimulated with anti-CD3/CD28-coated beads for 24 h. The supernatants were directly assayed for IL-2 concentration using the Human IL-2 Quantikine ELISA kit (R & D Systems, Inc., Minneapolis, MN). Results are given as ng/ml of secreted IL-2.
2.7 Phosphatase activity measurements
T cell lysate was prepared using lysis buffer described above, but without Na3VO4. The lysates were then diluted to a concentration of about 20,000 cells equivalents/μl. The hydrolysis of the general PTP substrate p-nitrophenyl phosphate (pNPP) was assayed at 30°C for 20–45 min in 0.15 M Bis-Tris, pH 6.0 assay buffer containing 1 mM dithiotreitol. The ionic strength was adjusted to 150 mM with NaCl. The reaction with 20 μl of lysate was initiated by addition of 4 mM pNPP to the reaction mixtures to a final volume of 100 μl. The reaction was quenched by addition of 100 μl 1 M NaOH. The nonenzymatic hydrolysis of the substrate was controlled for by including heat-inactivated samples (negative controls). The amount of p-nitrophenol product was determined from the absorbance at 405 nm detected by an EL×808 microplate reader (Bio-Tek Instruments, Inc.), using a molar extinction coefficient of 18,000 M−1cm−1.
2.8 Confocal microscopy
Immunofluorescence staining was performed as before (Gjörloff-Wingren et al., 2000; Nika et al., 2006) and the cells viewed under a Radiance 2100 MP (Bio-Rad, Hercules, CA) confocal laser scanning microscope. A differential interference contrast image was also taken of each cell.
2.9 Isolation of lipid rafts using sucrose gradient centrifugation
Lipid rafts were isolated from total cell lysates as described previously (Nika et al., 2006). One to two hundred million of human primary T cells were stimulated or unstimulated, and lysed on ice in 1 ml HNE buffer (50 mM HEPES [pH 7.4], 150 mM Sodium chloride, 5 mM EDTA, 1% Triton X-100, 5 mM Sodium orthovanadate, 10 mM Sodium pyrophosphate, 50 mM Sodium fluoride, and 1 mM PMSF) for 15 min, subjected to 10 rounds of Dounce homogenization, and mixed with an equal amount of 80% sucrose in HNE buffer. The mixed lysates were transferred to the bottom of Beckman ultracentrifuge tubes and were overlaid with 2 ml 30% sucrose in HNE buffer and then with 1 ml 5% sucrose in HNE buffer. The samples were ultracentrifuged for 20 h at 46,000 rpm in the Beckman SW55Ti rotor. Nine or ten fractions of 0.5 ml were collected from the top of the gradient.
3. Results
3.1. PTP-PEST is present in resting primary T cells, but not in Jurkat T leukemia cells
We began our work with PTP-PEST by confirming that this PTP is present in human T cells. Previous work showed that PTP-PEST is present in mouse thymocytes and splenic T cells (Davidson and Veillette, 2001). First, we analyzed human tissues for the presence of PTP-PEST mRNA by RT-PCR using a panel of cDNA libraries. A band of the expected size was readily obtained from most tissues, including spleen and thymus (Fig. 1A). Liver, skeletal muscle, and tonsils contained much less PTP-PEST mRNA. Immunoblotting with anti-PTP-PEST antibodies also revealed the presence of the 110–115-kDa PTP-PEST in mouse spleen and thymus (Fig. 1B, lanes 1 and 2). Freshly isolated human peripheral blood T cells also contained readily detectable levels of PTP-PEST (lane 3), while human T cells activated for 2 days with anti-CD3 plus anti-CD28 contained much less PTP-PEST (lane 4). In these experiments, we also noted that human PTP-PEST migrated slightly faster than mouse PTP-PEST. Finally, Jurkat T cells (both clone E6.1 and J-TAg) were negative for PTP-PEST (lane 5), even on very long exposures of the blots. PTP-PEST co-immunoprecipitated with Csk (Fig. 1C), as reported before (Davidson et al., 1997), and was largely cytoplasmic, as judged by immunofluorescence staining (Fig. 1D).
FIGURE 1. Expression of PTP-PEST in lymphoid cells.
A, PTP-PEST mRNA expression in multiple tissue cDNA libraries examined by PCR. B, Upper panel, detection of PTP-PEST protein by immunoblotting with a specific anti-PTP-PEST antibody in lysates from C57BL/6 mouse thymus (lane 1) and spleen (lane 2), freshly isolated primary human blood T lymphocytes (lane 3), primary human T lymphocytes activated with anti-CD3ε plus anti-CD28 for 2 days (lane 4), Jurkat T cells (lane 5). Lower panel, anti-actin immunoblot of the same filter. C, Anti-Csk immunoblotting using a mAb of immunoprecipitates obtained from primary human T cells using anti-Csk polyclonal antibody (lane 1), anti-PEST (lane 2), a rabbit preimmune serum (lane 3), or total lysates (lane 4). D, Immunofluorescence staining of Jurkat T cells transfected with PTP-PEST expression plasmid with rabbit polyclonal anti-PTP-PEST Ab. DIC, differential interference contrast.
3.2. Downregulation of PTP-PEST upon T cell activation and in memory T cells
When freshly isolated primary human T cells were stimulated for various times with anti-CD3/CD28 mAbs-coated beads, and then immunoblotted for PTP-PEST, we observed that PTP-PEST levels began to decrease at 12–24 h and reached less than <10% by 24–48 h, and then remained at this level for several days regardless of whether the cells were left in medium or were restimulated with anti-CD3 plus anti-CD28 (Fig. 2A and B). Addition of cytokines like IL-7 or IL-15 had no effect on PTP-PEST expression (not shown). The downregulation of PTP-PEST was dependent on the dose of anti-CD3, but was not affected by co-ligation of CD28, even at low doses of anti-CD3 (not shown).
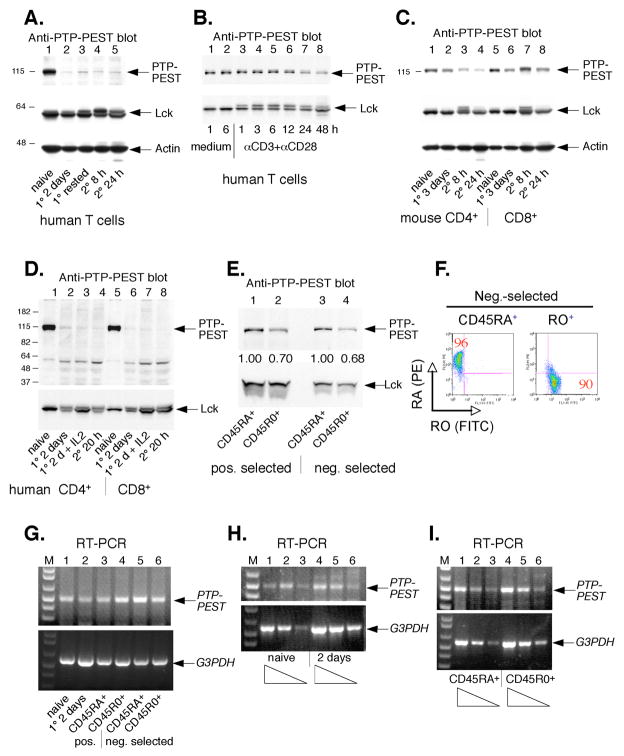
FIGURE 2. Downregulation of PTP-PEST upon T cell activation.
A Upper panel, anti-PTP-PEST immunoblot of freshly isolated primary human T cells (lane 1), the same cells stimulated with anti-CD3 plus anti-CD28 for 2 days (lane 2), the same cells rested for 2 additional days in medium with IL-2 (lane 3), the same cells restimulated with anti-CD3 plus anti-CD28 for 8 h (lane 4) or 24 h (lane 5). Middle panel, anti-Lck immunoblot of the same samples. Bottom panel, anti-actin blot of the same filter. B, Similar experiment with human T cells incubated for shorter times with anti-CD3 plus anti-CD28. C, Similar experiment with mouse CD4+ (lanes 1–4) and CD8+ (lanes 5–8) T cells, as indicated. D, Similar experiment with human CD4+ (lanes 1–4) and CD8+ (lanes 5–8) T cells. E, Anti-PTP-PEST immunoblot of human CD45RA+ naïve and CD45RO+ memory T cells purified positively (lanes 1 and 2) or negatively (lanes 3 and 4). The relative densitometry scores normalized against Lck are given below. Each CD45RA+ score is assigned as 1.00. F, FACS profile of purified T cells in Fig. 2E. G, RT-PCR for human PTP-PEST and control G3PDH from each T cell population, as indicated. H, Semi-quantitative RT-PCR for human PTP-PEST and G3PDH using serially 5-fold diluted cDNA template from fresh primary T cells (lanes 1–3), the same cells stimulated with anti-CD3 plus anti-CD28 for 2 days (lanes 4–6). I, Similar experiment with human CD45RA+ naïve T cells (lanes 1–3) or CD45RO+ memory T cells (lanes 4–6).
Freshly isolated C57BL6 mouse CD4+ and CD8+ T cells also contained readily detectable PTP-PEST protein (Fig. 2C). Upon activation with anti-CD3 plus anti-CD28, mouse CD4+ T cells also down-regulated PTP-PEST expression, albeit less dramatically than human T cells, while the expression levels changed much less in mouse CD8+ T cells upon stimulation and restimulation. In contrast, both human CD4+ and CD8+ T cells downregulated PTP-PEST very dramatically upon anti-CD3 stimulation (Fig. 2D).
To determine if previously activated ‘memory’ T cells isolated from healthy donors also display reduced PTP-PEST levels, we purified human T cells and sorted them negatively or positively by CD45R0 versus CD45RA antibodies and magnetic beads. Immunoblotting of lysates from the resulting populations showed that PTP-PEST levels were 30–32% lower in the CD45R0+ ‘memory’ T cells than in the CD45RA+ naïve T cells (Fig. 2E). The purity of the two populations was over 90% (Fig. 2F). Thus, PTP-PEST downregulation appears to occur also in under physiological conditions in humans.
3.3. No change in PTP-PEST mRNA despite loss of PTP-PEST protein
To determine whether the reduced levels of PTP-PEST protein in activated T cells was due to transcriptional silencing of the PTPN12 gene, we compared the levels of PTP-PEST mRNA between resting and activated T cells and found no differences at all in several consecutive experiments (Fig. 2G). In contrast, Jurkat T cells did not contain detectable PTP-PEST mRNA. A more careful quantitation of the levels of PTP-PEST mRNA in resting versus activated and in CD45RA+ naïve versus CD45R0+ memory T cells also failed to detect any differences in the mRNA (Figs. 2H and I). However, immunoblots of the same samples showed the dramatic loss of PTP-PEST protein, as in Fig. 2D and E. We conclude that the downregulation of PTP-PEST upon T cell activation is either translational or post-translational. Although a proteasome inhibitor MG132 did not have any effect on the low levels of PTP-PEST in activated T cells, Caspase inhibitors tended to rescue the expression partially in our preliminary experiment (data not shown) as reported recently (Halle et al., 2007).
3.4. Expression of PTP-PEST inhibits TCR-induced gene transactivation
To begin to evaluate the role of PTP-PEST in TCR signaling and T cell activation, and the consequences of its down-regulation upon T cell activation, we first determine how much PTP-PEST plasmid to use with E6.1 Jurkat T leukemia cells in order to achieve levels of expression that are similar to those in resting primary human T cells (Fig. 3A). PTP-PEST was then expressed together with luciferase reporter constructs that reflect the transactivation of TCR-regulated genes. TCR plus CD28 triggering of the transfected cells demonstrated that PTP-PEST very efficiently reduced the activation of the interleukin-2 promoter, the NFAT/AP-1 element from this gene, and, to a lesser extent, an NF-κB-driven reporter (Fig. 3B). As a control for specificity, we also expressed another large PTP found in T cells, HD-PTP (PTPN23) (Cao et al., 1998), which has a similar large Pro-rich non-catalytic portion of unknown structure, and is a catalytically active phosphatase in vitro (not shown). HD-PTP caused a minimal reduction in IL-2-luciferase activation, but had no significant effects on the other reporter genes.
FIGURE 3. PTP-PEST inhibits T cell activation.
A, Upper panel, anti-PTP-PEST immunoblot of freshly isolated primary human T cells (lanes 1 and 5), the same cells stimulated with anti-CD3 plus anti-CD28 for 3 days (lanes 2 and 6), Jurkat T cells transfected with 10 μg of control empty vector (lanes 3, 7, and 9) or 10 μg of PTP-PEST expression vector (lanes 4, 8, 10). Lower panel, anti-Lck blot of the same filter. Three independent experiments are shown. B, Induction of luciferase activity by anti-CD3ε plus anti-CD28 mAbs in Jurkat T cells transfected with PTP-PEST or HD-PTP expression plasmids together with the indicated luciferase reporter constructs. The data represent the average ± SD from triplicate determinations in one experiment and is representative of 3 independent experiments. The insert is an anti-PTP-PEST immunoblot of the lysates of the transfectants. C, Production of IL-2 by naïve/resting (left panel, “Naïve”) and once activated effector/memory (right panel, “memory”) primary human T lymphocytes nucleofected with the indicated constructs. The inserts are an anti-PTP-PEST immunoblot of the lysates of the same cells and an anti-Lck immunoblot as loading control. The result shown is a representative of two independent experiments.
Since once activated primary T cells down-regulate PTP-PEST, we decided to re-express PTP-PEST in these cells to determine if this would influence TCR signaling in a similar manner. Indeed, catalytically active PTP-PEST reduced IL-2 production by these cells to less than half (Fig. 3C, right panel), while the inactive Cys-to-Ser mutated PTP-PEST-CS had only a small effect. In contrast, expression of PTP-PEST in naïve resting T cells, which contain a good level of endogenous PTP-PEST (Fig. 2A and D), had little effect on IL-2 production (Fig. 3C, left panel).
3.5. PTP-PEST reduces Lck phosphorylation at Y394 and downstream signaling
Since PTP-PEST associates with Csk (Fig. 1C) (Davidson et al., 1997) and, unlike any other PTP, was found in a yeast screen for genes able to suppress the lethality of Src (Superti-Furga et al., 1996), we decided to determine if PTP-PEST acts on the Src family kinase Lck in T cells. PTP-PEST was transfected into Jurkat T cells at levels that approximate the endogenous levels of PTP-PEST in primary T cells and then assessed for its ability to dephosphorylate Lck at its activation loop site, Y394, by immunoblotting with a phospho-specific antibody. To put the effect of PTP-PEST in better perspective, we expressed several other PTPs known to affect early TCR signaling events (Mustelin et al., 2002, 2005) in parallel samples of Jurkat T cells. In these experiments, PTP-PEST was very efficient and essentially eliminated any phosphorylation of Lck at Y394 (Fig. 4A, lanes 4–6). As expected, LYP, PTPH1, and SHP1 also caused some reduction in Lck-Y394 phosphorylation, but they were not nearly as efficient as PTP-PEST, despite being well expressed (Fig. 4B). Inhibition of PTPs by addition of 5 mM sodium orthovanadate (the lysis buffer also contains vanadate) prevented the dephosphorylation of Lck by PTP-PEST (Fig. 4A, middle panel). The total amount of Lck was unchanged (Fig. 4A, lower panels). As another control, we blotted the same lysates with antibodies against the activated phospho-form of Erk and found it to be reduced by PTP-PEST, as well as the other PTPs, particularly by HePTP, which directly dephosphorylates Erk (saxena et al., 1998, 1999a, b; Saxena and Mustelin, 2000; Nika et al., 2006). Finally, to further show that the effect of PTP-PEST was not simply the consequence of overexpression of a PTP, we measured the total PTP activity of the lysates (Fig. 4C). While the transfected PTP-PEST made a noticeable contribution to the total PTP activity somewhat, LMPTP-B and HePTP caused much larger increases in total PTP activity. We have also confirmed that our LYP, PTPH1, and SHP1 constructs encode catalytically active enzymes (but each subject to its own regulation in cells). Nevertheless, PTP-PEST was much more efficient in dephosphorylating Lck at Y394, indicating that this reflects an intrinsic preference of PTP-PEST.
FIGURE 4. PTP-PEST dephosphorylates Lck at Y394 more efficiently than other T cell-expressed PTPs.
A, Upper panels, anti-Lck-pY394 immunoblot of lysates of Jurkat T cells transfected with empty vector (lanes 1–3) or expression plasmids for PTP-PEST (lanes 4–6), LYP (lanes 7–9), PTPH1 (lanes 10–12), SHP1 (lanes 13–15), LMPTP-B (lanes 16–18), or HePTP (lanes 19–21), and stimulated with anti-CD3ε for 0, 3, or 7 min, as indicated. Middle panels, same experiment including 5 mM sodium orthovanadate to block PTPs. Lower panels, anti-Lck blot to show total amounts of Lck. B, Control immunoblots for the same experiments, anti-PTP-PEST blot (upper panels), anti-HA immunoblot to show the other PTPs (middle panels), and anti-phospho-Erk blot (lower panels). C, PTP activity of the lysates of the same transfectants to show that the effects of PTPs does not correlate with total activity.
3.6. PTP-PEST inhibits downstream signaling events
Dephosphorylation of Lck at its activation loop site may explain how PTP-PEST inhibits T cell activation. This dephosphorylation is expected to result in decreased phosphorylation of downstream substrates for Lck and decreased activity of protein kinases that are activated by Lck. In fact, many of the reported substrates of PTP-PEST (e.g. WASp, paxillin, or p130Cas), may be indirectly affected by PTP-PEST. Indeed, when lysates of PTP-PEST transfected T cells were immunoblotted with anti-pTyr mAbs, it was clear that many, if not all, tyrosine phosphorylated proteins contained less pTyr than in cells transfected with empty vector or with HDPTP (Fig. 5A and B). This decrease was best appreciated in T cells triggered through their TCR for 3 or 6 min, but was also detectable in resting T cells, and it paralleled the decrease in Lck Y394 phosphorylation (Fig. 5A, lower panel). Blotting of lysates from Jurkat T cells transfected with PTP-PEST with a series of phospho-specific antibodies also revealed that ZAP-70 was much less phosphorylated at Y319 and Y493 (Fig. 5B, second and third panels), and that Erk and p38 MAP kinases were less phosphorylated in their activation loop (Fig. 5B, fourth and fifth panels). In contrast, catalytically inactive PTP-PEST-CS had no effects on these parameters of TCR signaling, indicating that PTP-PEST must be catalytically active to temper TCR signaling.
FIGURE 5. Downstream effects of PTP-PEST.
A, Upper panel, anti-pTyr immunoblot of Jurkat T cells transfected with empty vector (lanes 1–3), or expression plasmids for HDPTP (lanes 4–6) or PTP-PEST (lanes 7–9), and stimulated with anti-CD3ε for 0, 3, or 6 min, as indicated. Lower panel, anti-Lck-pY394 immunoblot of the same filter. B, Top panel, anti-pTyr immunoblot of Jurkat T cells transfected with empty vector (lanes 1 and 2), or expression plasmids for PTP-PEST (lanes 3 and 4) or catalytically inactive PTP-PEST-CS (lanes 5 and 6), and stimulated with anti-CD3ε for 0 or 3 min, as indicated. Second to eight panels, immunoblots of the same samples with the indicated antibodies.
3.7. Lipid rafts localization of a small fraction of PTP-PEST independently of Csk
Because PTP-PEST associates with SH3 domain of Csk kinase (Fig. 1C) (Davidson et al., 1997), we tried to determine PTP-PEST subcellular localization in terms of a lipid raft. As shown in Fig. 6A, up to 11% PTP-PEST was localized in lipid rafts in human primary naïve/resting T cells. A small fraction of Csk also was present in lipid rafts. However, 2–5 min after TCR stimulation, Csk dissociated from lipid rafts, while the PTP-PEST level in rafts did not change (Fig. 6B). Anti-LAT and anti-phospho-LAT blots showed that lipid raft fractionation and TCR stimulation worked very well. These results indicated that a small portion of PTP-PEST was in lipid rafts, but its recruitment was likely independent of Csk behavior.
FIGURE 6. Lipid raft localization of a small portion of PTP-PEST.
A, Left panel, immunoblots of fractionated lysates of non-stimulated human primary T cells by sucrose gradient centrifuge to show lipid raft fractions (fraction #2–3), as indicated. Numerals under anti-PTP-PEST blot indicate the percentage of PTP-PEST present in each fraction by densitometry analysis. B, Right panel, immunoblots of the pooled lipid raft fractions (#2–3) stimulated with anti-CD3ε plus secondary anti-mouse Ig for 0, 2, 5 min, as indicated.
4. Discussion
To the best of our knowledge, PTP-PEST is the first PTP found to be downregulated upon T cell activation. Perhaps surprisingly, several other PTPs, such as CD45 (Sanders et al., 1988), HePTP (Adachi et al., 1992), as well as PTPH1, PTP-MEG2, and HDPTP (our unpublished observations), are upregulated substantially after TCR stimulation. This suggested to us that PTP-PEST may be a gatekeeper of TCR signaling in naïve T cell activation, and that downregulation of PTP-PEST may be instrumental for the increased responsiveness of previously activated ‘memory’ T cells. Indeed, compared to several other T cell-expressed nonreceptor PTPs, PTP-PEST was remarkably efficient in reducing the activating tyrosine phosphorylation of the Lck kinase, as well as all measured downstream events. Thus, it seems likely that the TCR-induced decrease in PTP-PEST expression would facilitate the phosphorylation and activation of Lck and thereby augment downstream pathways in a second round of TCR stimulation.
It is well documented that re-stimulation of T cells that have gone through one cycle of activation, followed by a day or two of rest in medium (with or without IL-2), respond much more vigorously and rapidly to a second TCR stimulation. In particular, tyrosine phosphorylation begins faster and reaches higher levels than in naïve T cells, and cytokine production commences much earlier. The molecular mechanisms underlying this change in response magnitude and time-course are not known, but presumably include altered expression levels of key signaling molecules. Since signaling is enhanced from the very receptor-proximal step of Lck activation, and Lck levels do not appear to change measurably, it would be logical to assume that a key negative regulator of Lck has been downregulated. PTP-PEST fits this description very well: we find it to affect Lck Y394 phosphorylation much better than the PTPs previously implicated in Lck dephosphorylation (Fig. 4). Furthermore, we do not find any decrease in CD45, SHP1, or LYP expression during primary stimulation of freshly isolated human T cells, CD45 levels even appear to increase. Thus, the loss of PTP-PEST is most likely responsible for the increased Lck phosphorylation and activation in secondary T cells. In support of this notion, we find that expression of low levels of PTP-PEST in these cells converts their response to one typical of naïve T cells. On the other hand, when we tried to knock down endogenous PTP-PEST in human naïve T cells by using siRNAs, which gave a 50–60% reduction in the expression level, we failed to detect the significant difference in T cell responses (data not shown). We do not know yet the reason, but assume that a knockdown of multiple PTPs might be required.
The downregulation of PTP-PEST upon TCR stimulation is probably highly relevant for the phenotype of the mouse knockout for LYP (called PEP in the mouse), which stems from secondary stimulation of T cells (Hasegawa et al., 2004), i.e. the naïve T cells, which express both LYP and PTP-PEST, appear to be normal, while the effector/memory T cells that have downregulated PTP-PEST respond in an exaggerated and prolonged manner (Sanders et al., 1988). This would support the view that loss of LYP/PEP can be compensated for by PTP-PEST in naïve T cells and first responses, but not in memory T cells upon restimulation because PTP-PEST has been downregulated.
Finally, since a single-nucleotide polymorphism in position 1858 in PTPN22 (that encodes LYP) has been found to predispose carriers to multiple autoimmune diseases (Bottini et al., 2004; Smyth et al., 2004; Begovich et al., 2004; Viken et al., 2005; Kyogoku et al., 2004; Vang et al., 2005; reviewed in Bottini et al., 2006), it seems quite possible that similar variants of PTP-PEST may be involved in immunological disease (Mustelin, 2006). It is therefore important to clarify the regulation and physiological functions of these phosphatases in T lymphocytes and other immune cells.
Acknowledgments
We are grateful to Drs. J.-F. Cote and M. Tremblay for the rabbit polyclonal anti-PTP-PEST Ab and PTP-PEST expression vectors. This work was supported by grants AI53585, and AI35603 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adachi M, Sekiya M, Isobe M, Kumura Y, Ogita Z, Hinoda Y, Imai K, Yachi A. Molecular cloning and chromosomal mapping of a human protein-tyrosine phosphatase LC-PTP. Biochem Biophys Res Commun. 1992;186:1607–1615. doi: 10.1016/s0006-291x(05)81592-x. [DOI] [PubMed] [Google Scholar]
- Angers-Loustau A, Cote JF, Charest A, Dowbenko D, Spencer S, Lasky LA, Tremblay ML. Protein tyrosine phosphatase-PEST regulates focal adhesion disassembly, migration, and cytokinesis in fibroblasts. J Cell Biol. 1999;144:1019–1031. doi: 10.1083/jcb.144.5.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badour K, Zhang J, Shi F, Leng Y, Collins M, Siminovitch KA. Fyn and PTP-PEST-mediated regulation of Wiskott-Aldrich syndrome protein (WASp) tyrosine phosphorylation is required for coupling T cell antigen receptor engagement to WASp effector function and T cell activation. J Exp Med. 2004;199:99–111. doi: 10.1084/jem.20030976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begovich AB, Carlton VE, Honigberg LA, Schrodi SJ, Chokkalingam AP, Alexander HC, Ardlie KG, Huang Q, Smith AM, Spoerke JM, Conn MT, Chang M, Chang SY, Saiki RK, Catanese JJ, Leong DU, Garcia VE, McAllister LB, Jeffery DA, Lee AT, Batliwalla F, Remmers E, Criswell LA, Seldin MF, Kastner DL, Amos CL, Sninsky JJ, Gregersen PK. A Missense single-nucleotide polymorphism in a gene encoding a protein tyrosine phosphatase (PTPN22) is associated with rheumatoid arthritis. Am J Hum Genet. 2004;75:330–337. doi: 10.1086/422827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottini N, Musumeci L, Alonso A, Rahmouni S, Nika K, Rostamkhani M, MacMurray J, Meloni GF, Lucarelli P, Pellecchia M, Eisenbarth GS, Comings D, Mustelin T. A functional variant of lymphoid tyrosine phosphatase is associated with type I diabetes. Nat Genet. 2004;36:337–338. doi: 10.1038/ng1323. [DOI] [PubMed] [Google Scholar]
- Bottini N, Stefanini L, Williams S, Alonso A, Merlo J, Jascur T, Abraham RT, Couture C, Mustelin T. Activation of ZAP-70 through specific dephosphorylation at the inhibitory Tyr-292 by the low molecular weight phosphotyrosine phosphatase (LMPTP) J Biol Chem. 2002;277:24220–24224. doi: 10.1074/jbc.M202885200. [DOI] [PubMed] [Google Scholar]
- Bottini N, Vang T, Cucca F, Mustelin T. Role of PTPN22 in type 1 diabetes and other autoimmune diseases. Semin Immunol. 2006;18:207–213. doi: 10.1016/j.smim.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Brockdorff J, Williams S, Couture C, Mustelin T. Dephosphorylation of ZAP-70 and inhibition of T cell activation by activated SHP1. Eur J Immunol. 1999;29:2539–2550. doi: 10.1002/(SICI)1521-4141(199908)29:08<2539::AID-IMMU2539>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Cao L, Zhang L, Ruiz-Lozano P, Yang Q, Chien KR, Graham RM, Zhou M. A novel putative protein-tyrosine phosphatase contains a BRO1-like domain and suppresses Ha-ras-mediated transformation. J Biol Chem. 1998;273:21077–21083. doi: 10.1074/jbc.273.33.21077. [DOI] [PubMed] [Google Scholar]
- Cong F, Spencer S, Cote JF, Wu Y, Tremblay ML, Lasky LA, Goff SP. Cytoskeletal protein PSTPIP1 directs the PEST-type protein tyrosine phosphatase to the c-Abl kinase to mediate Abl dephosphorylation. Mol Cell. 2000;6:1413–1423. doi: 10.1016/s1097-2765(00)00138-6. [DOI] [PubMed] [Google Scholar]
- Cote JF, Charest A, Wagner J, Tremblay ML. Combination of gene targeting and substrate trapping to identify substrates of protein tyrosine phosphatases using PTP-PEST as a model. Biochemistry. 1998;37:13128–13137. doi: 10.1021/bi981259l. [DOI] [PubMed] [Google Scholar]
- Cote JF, Chung PL, Theberge JF, Halle M, Spencer S, Lasky LA, Tremblay ML. PSTPIP is a substrate of PTP-PEST and serves as a scaffold guiding PTP-PEST toward a specific dephosphorylation of WASP. J Biol Chem. 2002;277:2973–2986. doi: 10.1074/jbc.M106428200. [DOI] [PubMed] [Google Scholar]
- Cote JF, Turner CE, Tremblay ML. Intact LIM 3 and LIM 4 domains of paxillin are required for the association to a novel polyproline region (Pro 2) of protein-tyrosine phosphatase-PEST. J Biol Chem. 1999;274:20550–20560. doi: 10.1074/jbc.274.29.20550. [DOI] [PubMed] [Google Scholar]
- Davidson D, Cloutier JF, Gregorieff A, Veillette A. Inhibitory tyrosine protein kinase p50csk is associated with protein-tyrosine phosphatase PTP-PEST in hemopoietic and non-hemopoietic cells. J Biol Chem. 1997;272:23455–23462. doi: 10.1074/jbc.272.37.23455. [DOI] [PubMed] [Google Scholar]
- Davidson D, Veillette A. PTP-PEST, a scaffold protein tyrosine phosphatase, negatively regulates lymphocyte activation by targeting a unique set of substrates. EMBO J. 2001;20:3414–3426. doi: 10.1093/emboj/20.13.3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garton AJ, Flint AJ, Tonks NK. Identification of p130cas as a substrate for the cytosolic protein tyrosine phosphatase PTP-PEST. Mol Cell Biol. 1996;16:6408–6418. doi: 10.1128/mcb.16.11.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjörloff-Wingren A, Saxena M, Han S, Wang X, Alonso A, Renedo M, Oh P, Williams S, Schnitzer J, Mustelin T. Subcellular localization of intracellular protein tyrosine phosphatases in T cells. Eur J Immunol. 2000;30:2412–2421. doi: 10.1002/1521-4141(2000)30:8<2412::AID-IMMU2412>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Halle M, Liu YC, Hardy S, Theberge JF, Blanchetot C, Bourdeau A, Meng TC, Tremblay ML. Caspase-3 regulates catalytic activity and scaffolding functions of the protein tyrosine phosphatase PEST, a novel modulator of the apoptotic response. Mol Cell Biol. 2007;27:1172–1190. doi: 10.1128/MCB.02462-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa K, Martin F, Huang G, Tumas D, Diehl L, Chan AC. PEST domain-enriched tyrosine phosphatase (PEP) regulation of effector/memory T cells. Science. 2004;303:685–689. doi: 10.1126/science.1092138. [DOI] [PubMed] [Google Scholar]
- Klausner RD, Samelson LE. T cell antigen receptor activation pathways: The tyrosine kinase connection. Cell. 1991;64:875–878. doi: 10.1016/0092-8674(91)90310-u. [DOI] [PubMed] [Google Scholar]
- Kyogoku C, Langefeld CD, Ortmann WA, Lee A, Selby S, Carlton VEH, Chang M, Ramos P, Baechler EC, Batliwalla FM, Novitzke J, Williams AH, Gillett C, Rodine P, Graham RR, Ardlie KG, Gaffney PM, Moser KL, Petri M, Begovich AB, Gregersen PK, Behrens TW. Genetic association of the R620W polymorphism of protein tyrosine phosphatase PTPN22 with human SLE. Am J Hum Genet. 2004;75:504–507. doi: 10.1086/423790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons PD, Dunty JM, Schaefer EM, Schaller MD. Inhibition of the catalytic activity of cell adhesion kinase beta by protein-tyrosine phosphatase-PEST-mediated dephosphorylation. J Biol Chem. 2001;276:24422–24431. doi: 10.1074/jbc.M011080200. [DOI] [PubMed] [Google Scholar]
- Manie SN, Beck AR, Astier A, Law SF, Canty T, Hirai H, Druker BJ, Avraham H, Haghayeghi N, Sattler M, Salgia R, Griffin JD, Golemis EA, Freedman AS. Involvement of p130Cas and p105HEF1, a novel Cas-like docking protein, in a cytoskeleton-dependent signaling pathway initiated by ligation of integrin or antigen receptor on human B cells. J Biol Chem. 1997;272:4230–4236. doi: 10.1074/jbc.272.7.4230. [DOI] [PubMed] [Google Scholar]
- Monks C, Freiberg B, Kupfer H, Sciaky N, Kupfer A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature. 1998;395:82–86. doi: 10.1038/25764. [DOI] [PubMed] [Google Scholar]
- Mustelin T. Are other protein tyrosine phosphatases than PTPN22 associated with autoimmunity? Semin Immunol. 2006;18:254–260. doi: 10.1016/j.smim.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Mustelin T, Abraham RT, Rudd CE, Alonso A, Merlo JJ. Protein tyrosine phosphorylation in T cell signaling. Front Biosci. 2002;7:918–969. doi: 10.2741/A821. [DOI] [PubMed] [Google Scholar]
- Mustelin T, Vang T, Bottini N. Protein tyrosine phosphatases and the immune response. Nat Rev Immunol. 2005;5:43–57. doi: 10.1038/nri1530. [DOI] [PubMed] [Google Scholar]
- Nika K, Charvet C, Williams S, Tautz L, Bruckner S, Rahmouni S, Bottini N, Schoenberger SP, Baier G, Altman A, Mustelin T. Phosphorylation of hematopoietic protein tyrosine phosphatase (HePTP) at Ser-225 by protein kinase C θ in the T cell immune synapse. Mol Cell Biol. 2006;26:1806–1816. doi: 10.1128/MCB.26.5.1806-1816.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders ME, Makgoba MW, Shaw S. Human naive and memory T cells: reinterpretation of helper-inducer and suppressor-inducer subsets. Immunol Today. 1988;9:195–199. doi: 10.1016/0167-5699(88)91212-1. [DOI] [PubMed] [Google Scholar]
- Sastry SK, Lyons PD, Schaller MD, Burridge K. PTP-PEST controls motility through regulation of Rac1. J Cell Sci. 2002;115:4305–4316. doi: 10.1242/jcs.00105. [DOI] [PubMed] [Google Scholar]
- Saxena M, Mustelin T. Extracellular signals and scores of phosphatases: All roads lead to MAP kinase. Semin Immunol. 2000;12:387–396. doi: 10.1006/smim.2000.0219. [DOI] [PubMed] [Google Scholar]
- Saxena M, Williams S, Brockdorff J, Gilman J, Mustelin T. Inhibition of T cell signaling by MAP kinase-targeted hematopoietic tyrosine phosphatase (HePTP) J Biol Chem. 1999;274:11693–11700. doi: 10.1074/jbc.274.17.11693. [DOI] [PubMed] [Google Scholar]
- Saxena M, Williams S, Taskén K, Mustelin T. Crosstalk between cAMP-dependent kinase and MAP kinase through hematopoietic protein tyrosine phosphatase (HePTP) Nat Cell Biol. 1999;1:305–311. doi: 10.1038/13024. [DOI] [PubMed] [Google Scholar]
- Saxena M, Williams S, Gilman J, Mustelin T. Negative regulation of T cell antigen receptor signaling by hematopoietic tyrosine phosphatase (HePTP) J Biol Chem. 1998;273:15340– 15344. doi: 10.1074/jbc.273.25.15340. [DOI] [PubMed] [Google Scholar]
- Schlaepfer DD, Hunter T. Integrin signaling and tyrosine phosphorylation: just the FAKs? Trends Cell Biol. 1998;8:151–157. doi: 10.1016/s0962-8924(97)01172-0. [DOI] [PubMed] [Google Scholar]
- Shen Y, Lyons P, Cooley M, Davidson D, Veillette A, Salgia R, Griffin JD, Schaller MD. The noncatalytic domain of protein-tyrosine phosphatase-PEST targets paxillin for dephosphorylation in vivo. J Biol Chem. 2000;275:1405–1413. doi: 10.1074/jbc.275.2.1405. [DOI] [PubMed] [Google Scholar]
- Shen Y, Schneider G, Cloutier JF, Veillette A, Schaller MD. Direct association of protein-tyrosine phosphatase PTP-PEST with paxillin. J Biol Chem. 1998;273:6474–6481. doi: 10.1074/jbc.273.11.6474. [DOI] [PubMed] [Google Scholar]
- Smyth D, Cooper JD, Collins JE, Heward JM, Franklyn JA, Howson JMM, Vella A, Nutland S, Rance HE, Maier L, Barratt BJ, Guja C, Ionescu-Tirgoviste C, Savage DA, Dunger DB, Widmer B, Strachan DP, Ring SM, Walker N, Clayton DG, Twells RCJ, Gough SCL, Todd JA. Replication of an association between the lymphoid tyrosine phosphatase locus (LYP/PTPN22) with type 1 diabetes, and evidence for its role as a general autoimmunity locus. Diabetes. 2004;53:3020–3023. doi: 10.2337/diabetes.53.11.3020. [DOI] [PubMed] [Google Scholar]
- Sozio MS, Mathis MA, Young JA, Wälchli S, Pitcher LA, Wrage PC, Bartók B, Campbell A, Watts JD, Aebersold R, van Huijsduijnen RH, van Oers NSC. PTPH1 is a predominant protein tyrosine phosphatase capable of interacting with and dephosphorylating the T cell receptor z subunit. J Biol Chem. 2004;279:7760–7769. doi: 10.1074/jbc.M309994200. [DOI] [PubMed] [Google Scholar]
- Spencer S, Dowbenko D, Cheng J, Li W, Brush J, Utzig S, Simanis V, Lasky LA. PSTPIP: a tyrosine phosphorylated cleavage furrow-associated protein that is a substrate for a PEST tyrosine phosphatase. J Cell Biol. 1997;138:845–860. doi: 10.1083/jcb.138.4.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinchcombe JC, Bossi G, Booth S, Griffiths GM. The immunological synapse of CTL contains a secretory domain and membrane bridges. Immunity. 2001;15:751–761. doi: 10.1016/s1074-7613(01)00234-5. [DOI] [PubMed] [Google Scholar]
- Superti-Furga G, Jönsson K, Courtneidge SA. A functional screen in yeast for regulators and antagonizers of heterologous protein tyrosine kinases. Nat Biotechnol. 1996;14:600–605. doi: 10.1038/nbt0596-600. [DOI] [PubMed] [Google Scholar]
- Tautz L, Bruckner S, Sareth S, Alonso A, Bogetz J, Bottini N, Pellecchia M, Mustelin T. Inhibition of Yersinia tyrosine phosphatase by furanyl salicylate compounds. J Biol Chem. 2005;280:9400–9408. doi: 10.1074/jbc.M413122200. [DOI] [PubMed] [Google Scholar]
- Toyooka S, Ouchida M, Jitsumori Y, Tsukuda K, Sasaki A, Nakamura A, Shimizu N, Shimizu K. HD-PTP: A nobel protein tyrosine phosphatase gene on human chromosome 3p21.3. Biochem Biophys Res Commun. 2000;278:671–678. doi: 10.1006/bbrc.2000.3870. [DOI] [PubMed] [Google Scholar]
- Vang T, Congia M, Macis MD, Musumeci L, Orru V, Zavattari P, Nika K, Tautz L, Tasken K, Cucca F, Mustelin T, Bottini N. Autoimmune-associated lymphoid tyrosine phosphatase is a gain-of-function variant. Nat Genet. 2005;37:1317–1319. doi: 10.1038/ng1673. [DOI] [PubMed] [Google Scholar]
- Viken MK, Amundsen SS, Kvien TK, Boberg KM, Gilboe IM, Lilleby V, Sollid LM, Forre OT, Thorsby E, Smerdel A, Lie BA. Association analysis of the 1858C>T polymorphism in the PTPN22 gene in juvenile idiopathic arthritis and other autoimmune diseases. Genes Immun. 2005;6:271–273. doi: 10.1038/sj.gene.6364178. [DOI] [PubMed] [Google Scholar]
- Zhang S, Liu J, Kobayashi R, Tonks N. Identification of the cell cycle regulator VCP (p97/CDC48) as a substrate of the band 4.1-related protein-tyrosine phosphatase PTPH1. J Biol Chem. 1999;274:17806–17812. doi: 10.1074/jbc.274.25.17806. [DOI] [PubMed] [Google Scholar]
- Wu Y, Dowbenko D, Lasky LA. PSTPIP 2, a second tyrosine phosphorylated, cytoskeletal-associated protein that binds a PEST-type protein-tyrosine phosphatase. J Biol Chem. 1998;273:30487–30496. doi: 10.1074/jbc.273.46.30487. [DOI] [PubMed] [Google Scholar]