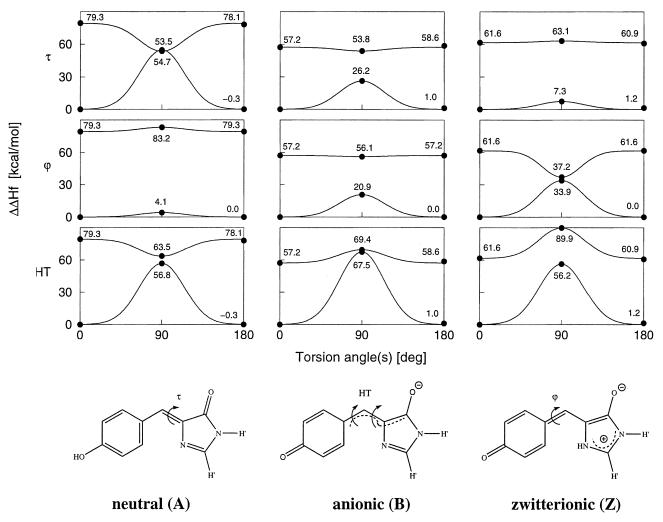
Figure 1.
Models of p-hydroxybenzylideneimidazolidinone GFP chromophore in the neutral, anionic, and zwitterionic forms used in the quantum chemical calculations, shown in those resonance structures that best represent the calculated bond orders. The covalent links with the Ser65 and Gly67 Cα atoms are replaced by hydrogen atoms (indicated as H′). The torsional degrees of freedom around the two ring-bridging bonds considered here are denoted by ϕ, τ (C—C—C—N), and HT (hula-twist (27), concerted and simultaneous motion around ϕ and τ), respectively. Rotation by 180° around ϕ leaves the structure unchanged. The configurations displayed represent τ = 0° and are referred to as cis configurations. The upper panels show OM2 (ref. 28; W.W. and W. Thiel, unpublished work)/PERTCI (29) energy profiles for rotation around the dihedral angles τ and ϕ and for the HT motion in the ground and first singlet excited states. Calculated values are marked by dots, and the Gaussian profiles are shown as visual aid. Note that, for these calculations, we fixed the dihedral angles while relaxing all other degrees of freedom.